At pressures of millions of atmospheres, hydrogen – normally an excellent insulator thanks to the tightly-bound electrons in the H2 molecule – becomes an electrical conductor. Its exact transition point is, however, the subject of much debate, with the results of several recent experiments seemingly contradicting each other. Researchers in Italy, Spain and France now say they have resolved the discrepancy by using an advanced computing technique that takes the quantum fluctuations of protons into account when simulating the behaviour of hydrogen at high pressures. The group’s simulations also reveal that hydrogen in this metal-like phase reflects very little light, and would thus appear pitch-black to anyone who observed it – further proof, says study lead author Lorenzo Monacelli, that metallic hydrogen is a very peculiar substance indeed.
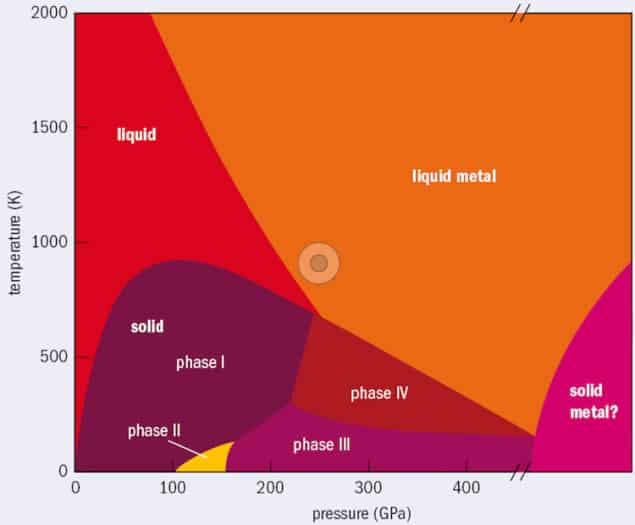
Solid hydrogen at high pressures boasts a rich phase diagram, with five insulating molecular phases labelled I to V. The idea that it might become metallic at high pressures can be traced back to a theoretical proposal made by the Hungarian-born physicist Eugene Wigner and his American colleague Hillard Bell Huntington in 1935. Since then, metallic hydrogen has been predicted to exist in the core of gas-giant planets like Jupiter and Saturn. It has also been suggested that metallic hydrogen could be a room-temperature superconductor.
Difficult to stabilize and challenging to characterize
Such predictions are hard to confirm, however, because metallic hydrogen is extremely difficult to stabilize in the laboratory. It is also challenging to characterize, since it does not interact with X-rays and techniques that rely on neutron scattering cannot be used at high pressures. Scientists must therefore infer its structure indirectly, using vibrational spectroscopy techniques like Raman and infrared spectroscopy or optical approaches that measure light transmittance and reflectivity from a sample.
These intense experimental difficulties may well explain why different research groups have obtained apparently contradictory results about the behaviour of hydrogen at low temperatures and high pressures. Optical reflectivity measurements suggest that metallization takes place in atomic hydrogen at 495 GPa, when the element transforms from a black lower-pressure phase to a shiny higher-pressure one. Electrical conductivity measurements, meanwhile, imply that hydrogen in phase III – a molecular phase in which the strength of the hydrogen-hydrogen bonds is thought to become so weak that protons can jump between different molecules – exhibits semi-metallic behaviour at pressures above 360 GPa. Infrared light transmission experiments, for their part, suggest that phase III hydrogen becomes metallic at 420 GPa.
Not contradictory
According to researchers led by Francesco Mauri of the University Sapienza in Rome, these results are not, in fact, contradictory. The team came to this conclusion by simulating the properties of phase III hydrogen under pressures of between 150 GPa and 450 GPa. In their simulations, they used a technique called the Self-Consistent Harmonic Approximation (SCHA) to account for quantum effects on the hydrogen nuclei. Simply put, this technique simulates the wavefunction of the hydrogen nuclei (protons) by sampling their probability cloud – that is, the collection of locations where nuclei are likely to be found. The researchers then solved the electrons’ quantum equations for each configuration of nuclei.
This approach means that nuclei are not treated as static “balls” frozen in position, as is the case in most simulations. Instead, the nuclei have delocalized wavefunctions, the square of which describe the probability of where they might be located at a given time.
Based on these simulations, the researchers found that phase III hydrogen starts conducting electricity at 370 GPa – a result that Monacelli says is “in very close agreement with previous experimental findings” – while reflecting very little light even at the highest pressures. They also found that factoring in quantum effects on nuclei has a dramatic impact on hydrogen’s physical properties. This impact was expected, since the nucleus of hydrogen (the lightest element) is subject to large quantum fluctuations, but Monacelli says that the difference was still impressive.
“We found that the bond length of the H2 molecules increases by 6% (which is huge in terms of the energy involved) if quantum effects are considered,” he explains. “The [simulated] Raman and infrared spectra completely change when these effects are taken into account, with signal peaks shifting by more than 25% and their energy profile broadening a lot.” Such simulations of vibrational spectra are important, he says, because they are “the only way we can understand the crystalline structure of high-pressure hydrogen”. Encouragingly, he adds that the team’s calculated results exhibited “a remarkable agreement” with results from experimental studies of infrared absorption.
Machine-learning study of metallic hydrogen provides clues about Jupiter’s interior
Very peculiar
Based on these results (which are detailed in Nature Physics), Monacelli says that if the data from the optical reflectivity experiments are correct, then the high measured reflectivities observed in those experiments are not consistent with hydrogen existing in a molecular phase. Instead, they imply that hydrogen is in its atomic form. “We have proven that molecular metallic hydrogen is very peculiar,” he tells Physics World. “It is conductive, black, and transparent in the infrared. This is almost unique for a metal.”
The calculations of Mauri, Monacelli and colleagues also led them to make a new prediction: deuterium – hydrogen’s heavier isotope, with a neutron as well as a proton in its nucleus – should start to conduct electricity at pressures 70 GPa higher than ordinary hydrogen. “This prediction could be confirmed in the coming months to further support or contradict our results,” Monacelli says.




