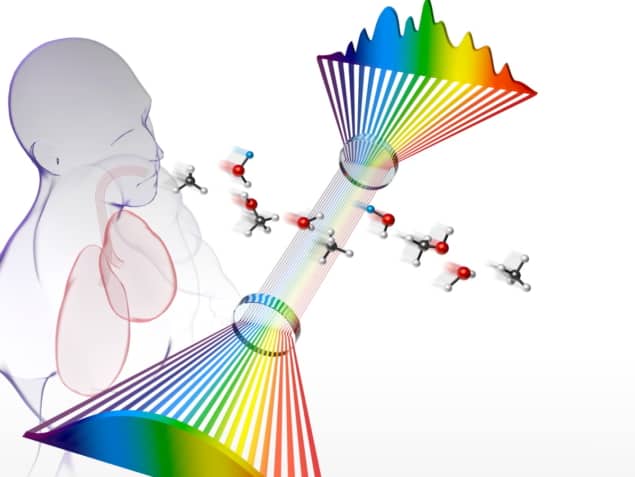
A team of US-based researchers has developed an innovative frequency comb breathalyser that is one thousand times more sensitive to disease biomarkers than the previous version – paving the way for substantial improvements in the use of non-invasive human breath analysis to detect and monitor disease.
The new breathalyser, created by scientists at JILA, University of Colorado Boulder and NIST – as well as the Center for Astrophysics, Harvard & Smithsonian – uses a mid-IR frequency comb to improve detection sensitivity by two orders of magnitude compared with a near-IR frequency comb made in the same lab in 2008. The team present the findings in the Proceedings of the National Academy of Sciences.
As corresponding author Jutta Toscano, postdoctoral researcher at the University of Basel (previously Lindemann fellow at JILA), explains, the new frequency comb, which is tuneable between 3 and 5 μm, allows the team to probe the molecular fingerprint region where fundamental, and more intense, spectroscopic transitions are found. It also enables users to probe a broadband spectrum instantaneously, without the need to scan the laser frequency – meaning that they can observe many molecules simultaneously without needing to compromise on resolution.
Besides probing the mid-IR region, where transitions are more intense, Toscano reveals that the team also enhanced the sensitivity by coupling the frequency comb into a high-finesse optical cavity.
“By matching the frequency of the comb teeth with the cavity modes – the ‘standing modes’ of the cavity – we can increase the interaction path length between molecules inside the cavity and laser light by a factor of around 4000, equivalent to an effective path length of a few kilometres,” Toscano says. “We then probe the light that leaks out of the cavity by sending it into an FTIR [Fourier-transform infrared] spectrometer to find out which exact comb teeth have been absorbed and by how much. In turn, this tells us which molecules are present in the breath sample and their concentration.”
Campus study
The team is currently using the new device to carry out a campus-wide study at the University of Colorado Boulder, as part of which it tests students’ breath and uses machine learning to look for correlations between the molecules present in breath and potential conditions affecting the participants – such as being covid-positive, for example, or suffering from asthma, diabetes or intestinal issues.
“After identifying these correlations, we would like to be able to reliably predict the presence or absence of these conditions from looking at the breath alone,” explains Toscano. “So far, the apparatus is not exactly compact or transportable, but our collaborators at NIST and CU Engineering – Scott Diddams and Greg Rieker, respectively – are looking into miniaturizing the frequency comb to make the breathalyser suitable for settings other than a laboratory.”
For Toscano, the main advantages of laser-based breathalysers like this include the collection speed, as well as the ability to easily differentiate between different isomers (molecules with the same mass but different structure, which would appear at the same mass-to-charge position in a mass spectrum) and between molecules of similar mass (which can be challenging to resolve in mass spectra).

Using breath to detect lung cancer
“On the other hand, most laser-based breathalysers operate at a single frequency to monitor a single molecule, with the notable exception of devices where a few single-frequency lasers are combined to monitor a few different molecules simultaneously,” she says.
“Adopting frequency combs as a laser source enables us to detect tens of molecules, or potentially even more, simultaneously, circumventing the potential complexity of having to combine tens of different laser sources. This is done by making use of the thousands of comb teeth that compose a frequency comb, which can be thought of as a collection of thousands of very narrow CW [continuous wave] lasers, each with an extremely well-defined frequency,” she adds.