Graphene might be the wonder kid of the materials world but other 2D nanosheets also show great promise for novel applications, says Belle Dumé
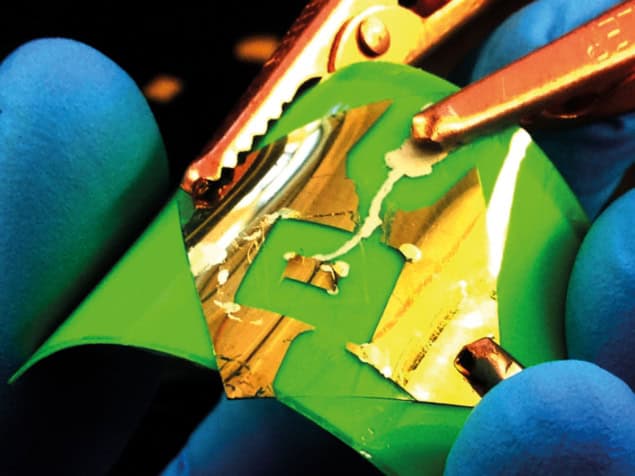
The unique electronic, optical, chemical and mechanical properties of 2D materials are creating a flurry of interest in laboratories around the world. Made up of individual atomic planes weakly held together by Van der Waals forces, these apparently simple systems behave very differently to their 3D counterparts and are therefore seen as a promising route for new electronic and other devices. The most widely studied 2D crystal is graphene: a planar sheet of carbon atoms arranged in a honeycomb lattice that is thinner and stronger than any other known material.
Since it was first isolated in 2004, graphene has continued to surprise. Some researchers believe that it might even become as important as silicon for the electronics industry. This is because electrons whizz through its 2D lattice at extremely high speeds, behaving like “Dirac” particles with no rest mass, which leads to extremely high conductivity. Graphene also shows great promise for photonics applications because it has an ideal internal quantum efficiency: almost every photon absorbed generates an electron–hole pair that could, in principle, be converted into electric current. And thanks to its Dirac electrons, graphene can also absorb light of any colour and has an extremely fast response, which could lead to much quicker optoelectronics devices for telecommunications.
However, graphene’s extreme conductivity is also a problem because the material remains conducting even when the power is switched off – wasting energy and preventing graphene components from being packed into computer chips as silicon components are today. There are other reasons why all is not plain sailing with this 2D wonder material. Although graphene is a semi-metal or “zero-gap” semiconductor, it is unlike familiar semiconductors such as silicon because it does not have an energy gap between its valence and conduction bands. Such a band gap allows a semiconductor to switch the flow of electrons on and off, which is the principle by which transistors operate. Researchers have proposed various schemes to overcome this problem, for example cutting graphene into nanoscale ribbons or chemically modifying the material to make it properly semiconducting, but such approaches damage the material and spoil its high electron mobility.
These drawbacks have turned researchers’ attention to 2D materials that naturally possess a band gap, such as transition-metal dichalcogenides (TMDCs), hexagonal boron nitride and layered oxides. These monolayer materials might even be combined with graphene to make novel hybrid heterostructures that have exceptional electronic and mechanical properties. “This new class of materials promises all: insulators, metals, semiconductors and superconductors,” says Sefaatin Tongay of Arizona State University in the US.
Semiconductor promise
TMDCs consist of a layer of transition-metal atoms sandwiched between two layers of chalcogen atoms, such as sulphur, selenium or tellurium, and they can be made using similar methods employed to obtain graphene. In bulk form, TMDCs are indirect band-gap semiconductors, but when scaled down to monolayers, the strong coupling between the neighbouring layers turns them into direct band-gap semiconductors. The material is therefore very efficient at absorbing and emitting light, and because TMDCs can be placed on a variety of substrates, they are ideal for optoelectronic devices such as LEDs and solar cells.
One much-studied TMDC, which is made from molybdenum and sulphur (MoS2), shows particular promise. But others discovered in the past few years include MoSe2, NbS2, ReSe2 and WSe2 (see table below). These materials could find similar applications as proposed for graphene or, if combined with graphene’s unique electronic and mechanical properties, could be used to make superior nanoelectronic circuits.
Earlier this year, however, Tongay and colleagues discovered a new 2D material called rhenium disulphide. Despite officially being a member of the semiconducting layered TMDC family, the material behaves as though it is a pure monolayer. Unlike other 2D materials though, it does not undergo an indirect-to-direct band-gap transition when scaled down to monolayers. The system therefore provides researchers with a 3D crystal in which they can study 2D phenomena without the difficulty of preparing large and high-quality monolayers.
Perhaps the next-best material to graphene in terms of mechanical and thermal properties is hexagonal boron nitride
Perhaps the next-best material to graphene in terms of mechanical and thermal properties is hexagonal boron nitride (hBN). Also known as “white graphene”, hBN is an ideal substrate for graphene because the two materials have very similar lattice constants. Unlike graphene, hBN is an insulator with a very large energy band gap, which means that monolayers of hBN integrated with graphene can be used as gate dielectrics and tunnel barriers with very few defects.
Indeed, hBN has particularly strong phonon resonances in the technologically important infrared band, which some physicists believe could be used to process information in nanodevices. “Flexible nanoelectronics could be the main application for the portfolio of 2D materials where graphene, TMDCs and hBN might be combined to make high-performance ultra-flexible transparent transistors on plastics and soft substrates,” says Deji Akinwande of the University of Texas at Austin.
Graphene derivative
Since graphene first rocked the materials world a decade ago, researchers have been exploring derivatives such as fluorographene, which is a wide-gap insulator made by fluorinating graphene. Similarly, the large band gaps in “graphane” and “graphone” (hydrogenated and semi-hydrogenated versions of graphene, respectively) could be used to make transistors with a large on–off current ratio, although researchers first need to find a way to prevent these materials from gradually losing their hydrogen atoms. Researchers are also exploring “graphynes”: 2D carbon allotropes built from double- and triply-bonded carbon atoms instead of just double bonds. These materials naturally contain conducting charge carriers and therefore could be made into semiconductors without the need for external doping.
According to some researchers, one of the most promising graphene-based derivatives is graphene oxide. This material is just like ordinary graphene but is covered with molecules such as hydroxyl groups or oxygen, which remove electronic states and turn the graphene into an insulator. Sheets of graphene oxide can easily be stacked on top of each other to form extremely thin but mechanically strong membranes, which could serve as molecular sieves. In addition to applications in water filtration and desalination, such systems might also be used for hydrogen storage, polymer solar cells, and flexible colour displays and smart textiles.
It is not yet clear whether graphene will live up to its promise and “win out” over other 2D materials, nor when the 2D-materials revolution will really start to affect our lives. According to Tongay, it is likely that there will be many winners in the race and that more competitors will appear relatively soon, such as phosphorene and silicene. “Graphene kick-started the 2D materials field and will remain an integral member of the 2D family, but the other 2D materials will bring new functionalities,” he says. “The 2D-materials revolution is here and I very much hope to see these materials integrated into our daily lives in different forms, from flexible electronics and solar cells to applications that we have not even dreamed of yet.”
The most promising 2D materials
Graphene family
Graphene – Extremely high conductivity and mechanical strength but no band gap in pristine state
Graphene oxide – Promising for molecular sieves, hydrogen storage and polymer solar cells
Graphane and graphone – Large on–off current ratio and large band gap but gradually lose hydrogen
Graphyne – Naturally contains conducting charge carriers
hBN (white graphene) – Good mechanical and thermal properties; an insulator and exceptional substrate for graphene
Fluro- and chlorographene – Often called the 2D version of Teflon, demonstrating insulating properties
BCN – Electronic properties ranging from insulating to semi-metallic
2D dichalcogenides
MoS2, WS2, MoSe2, WSe2 – Good at absorbing/emitting light and high charge mobility
Semiconducting dichalcogenides (e.g. MoTe2, WTe2, ZrS2, ZrSe2, ReS2) – Good for low-friction applications, such as lubricants
Metallic dichalcogenides (e.g. NbSe2, NbS2, TaS2, TiS2, NiSe2) – Some become superconductors below a certain temperature
Layered semiconductors (e.g. GaSe, GaTe, InSe, Bi2Se3) – Can be exfoliated onto a number of different substrates
2D oxides
Micas, BSCCO – Wide band gap; potential for all-oxide electronics, thermoelectrics and fuel cells
Layered copper oxides – Could become high-temperature superconductors when charge carriers are added to the layers
MoO3, WO3, SnS2, SnSe2, SnTe2 – Might be good for doping carbon nanotubes
TiO2, MnO2, V2O5, TaO3, RuO2 – Wide-ranging electronic, chemical and mechanical properties; has potential for thermoelectrics
Hydroxides (e.g. Ni(OH)2, Eu(OH)2) – Strong redox properties
Perovskite-type (e.g. LaNb2O7, Bi4Ti3O12, Ca2Ta2TiO10) – Good substrates for growing cuprates, colossal magnetoresistive manganites and multiferroics
Others
Silicene – Unstable as free-standing sheet
Phosphorene – Natural semiconductor but is difficult to produce in larger sheets
Germene – Natural semiconductor but hard to grow and environmental stability is unknown
(Courtesy: Adapted from A K Geim and I V Grigorieva Nature 499 419)
- For more information, see IOP Publishing’s new journal 2D Materials