Cartilage – the connective tissue that provides a smooth, lubricated surface between joints in the body – is a structural marvel, but its limited capacity for self-repair complicates injury treatment. To boost the healing process, researchers have long been keen to find new cell-based therapies, and have identified cartilage as a promising candidate for tissue engineering.
“This idea was supported by the apparent morphological simplicity of cartilage – a tissue composed of a single cell type, namely chondrocytes,” explains Wojciech Święszkowski, a biomaterials expert based at Warsaw University of Technology.
His team – which includes Andrea Barbetta and a group of chemists at Sapienza University of Rome, and specialists in stem-cell research at Oslo University Hospital directed by Jan Brinchmann – is helping to advance cartilage regeneration through the development of 3D-printed biomimetic hydrogel scaffolds. To fabricate these intricate matrix structures for supporting cell growth, the researchers have developed custom apparatus that provides microfluidic control over the dispensing of bioink through co-axial nozzles.
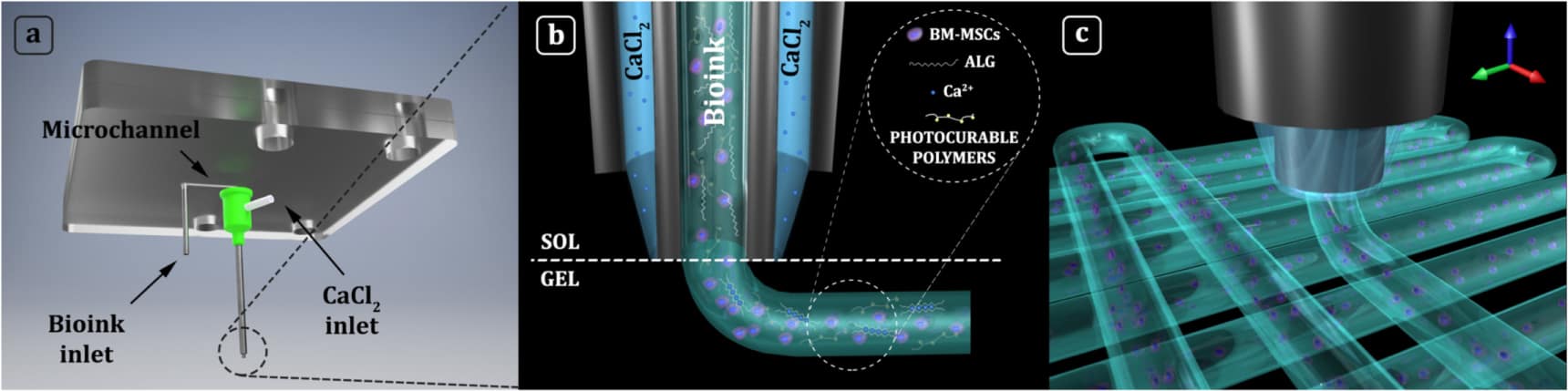
According to Święszkowski, who has presented the work in the journal Biofabrication, the set-up unlocks a new level of printing accuracy for extrusion-based systems. “Using this apparatus, we can deposit cells with precision beyond the dimension of a single laid fibre,” he explains. “It means that extrusions can contain multiple cell types or biomaterials, which allows us to pattern 3D constructs that more closely mimic the body’s own tissue.”
The technique also makes advances in decoupling printing accuracy and precision from bioink rheology. “We can freely change bioink composition in terms of biopolymer content and/or cellular density, without changing printing conditions such as printing speed, layer thickness, or the distance between fibres,” Święszkowski comments.
This flexibility gives the team more freedom to tailor the structure and composition of the fibre. In the case of cartilage repair, the group has succeeded in bioprinting biomimetic extracellular matrices composed of methacrylated derivatives of gelatin, hyaluronic acid and chondroitin sulphate.
Święszkowski’s team also has projects running that focus on repairing bone, skeletal muscle, tendon and pancreatic tissue. Examining the performance of their fabricated designs, the researchers have shown that three-dimensional constructs bioprinted using the co-axial extrusion system exhibited functional features after culturing in the lab.
In related work, the researchers have formulated and bioprinted a series of biomimetic inks containing human-bone-marrow-derived mesenchymal stem cells (hBM-MSCs), with the aim of examining the influence that each component exerts on cell differentiation. “Interestingly, we found that the composition and the stiffness of the printed bioink plays a key role in the differentiation of hBM-MSCs. We obtain the best results for a bioink composed of gelatin methacrylate and chondroitin sulphate amino ethyl methacrylate,” says Święszkowski.
How do you define biofabrication today?
The group is looking to apply its knowledge to a range of different scenarios, which includes the regeneration of skeletal muscle tissue. Noteworthy preliminary results – attained in collaboration with the group of Cesare Gargioli from University of Rome Tor Vergata – include bioprinting aligned layers of highly-packed hydrogel fibres containing skeletal muscle precursor cells (myoblasts) to create functionally organized and fully differentiated myobundles. “In the future, this 3D bioprinting approach could be used to assemble macroscopic constructs for treating volumetric muscle loss, a severe condition that can arise after injuries, traumas and degenerative disease,” Święszkowski points out.
The results are promising, but there are more challenges to be addressed. “Without any doubt, the major step for me and my team is to bring our system to the next level by improving the current apparatus and building superior structures that can better and more efficiently recreate the complexity and functions of the human body,” Święszkowski comments.
He adds that future progress requires focus not just on the deposition systems, but also on bioink development to expand the choice of natural polymers available for designs. Święszkowski feels that performance gains can be found by focusing on the cellular benefits offered by new blends of matrix materials.
At the same time, he believes that advances in protocols for stem cell differentiation together with progress in understanding key growth-factors and their stimuli will play a major role in delivering further advances in organ healing and tissue repair. “There is still a lot to do in the field of biofabrication and 3D bioprinting,” said Święszkowski, as he looks forward to tackling more challenges in 2018.
Full details of the team’s work can be found in the journal Biofabrication.
- This article is one of a series of reports reviewing progress on high-impact research originally published in the IOP Publishing journal Biofabrication.




