Transitioning molecular imaging from a preclinical technique to a standard clinical tool could help detect the very earliest stages of disease, reports Tami Freeman
Molecular imaging – the in vivo visualization of cellular processes – is used extensively within preclinical research. Positron emission tomography (PET), single photon emission computed tomography (SPECT), fluorescence imaging and other techniques with a high sensitivity can all non-invasively image molecular targets in living animals, shedding light on the origins and mechanisms of disease, and enabling potential new therapies to be tested over time.
But can these advantages be translated from preclinical studies to a clinical setting? “We’ve been using imaging in preclinical modes for two decades – it’s now time to take what we’ve learned and translate it to the clinic,” says Christopher Contag, principal investigator of the Molecular Biophotonics and Imaging Laboratory at Stanford University in the US.
Early diagnosis of diseases like cancer is vital for improving treatment outcomes
Making an early diagnosis of diseases like cancer is vital for improving treatment outcomes. Almost all diseases begin with alterations at the cellular level, and detecting these earliest changes calls for imaging techniques with both high sensitivity and high resolution.
Unfortunately, most of today’s diagnostic tools can find only relatively large lesions. For example, magnetic resonance imaging (MRI) and X-ray computed tomography (CT) can detect tumours of approximately 1 cm3 in volume, equivalent to about one billion cells, while methods based on blood tests can detect about one million cells. Optical-imaging techniques such as fluorescence microscopy, on the other hand, can detect lesions as small as 0.001 mm3 – equivalent to about 1000 cells. “To improve early diagnosis, we need tools that can detect microscopic lesions,” Contag says.
Point-of-care diagnosis
So could optical techniques provide this desired early detection? Optical imaging certainly offers the necessary high resolution, but light cannot penetrate far into the body, which is a problem. While MRI and CT scan through the entire body, techniques such as white-light endoscopy can only image up to 1 cm beneath the surface of the body. Confocal microscopy, meanwhile, offers 1 μm resolution at depths of just 100 μm to 1 mm.
It should be possible, however, to exploit existing methods such as endoscopy to place the optical-imaging tools directly at the tissue surface and visualize early changes with microscopic resolution. This approach could shift diagnostic methods from “biopsy followed by histopathology” to non-invasive “point-of-care” diagnosis. “We’d like to provide the pathologist with an in vivo image that looks a lot like a stained-tissue image,” Contag explains.
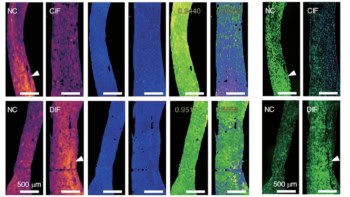
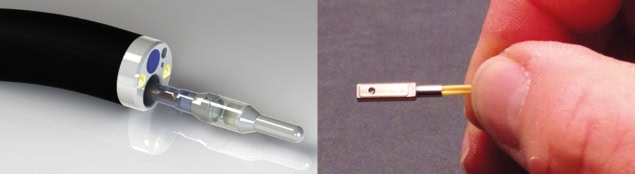
To do this, the Stanford team has developed a miniaturized dual-axis confocal fluorescence microscope with a MEMS (microelectromechanical systems)-based scanning core. The researchers showed that the microscope, which is small enough to fit inside the instrument channel of a standard endoscope, could image the junction between Barrett’s oesophagus (a condition that increases the risk of oesophageal cancer) and normal cells. This junction is currently detected by taking biopsies in random suspect areas; in vivo imaging could instead accurately reveal where best to biopsy.
The team is now testing the miniaturized microscopes in the clinic to image oesophageal and colon cancer, using topical indocyanine green (ICG) as a contrast agent. ICG acts as both a colorimetric and a fluorescent contrast, enabling simultaneous macroscopic viewing and microscopic imaging with 3–5 μm resolution.
Contag and colleagues have made several modifications to the original microscope design, including stitching together overlapping image frames in real time to increase the field of view. They have also redesigned the device to perform 3D imaging by using two MEMS mirrors to scan in the x–y and z directions – enabling scanning of a 300 μm3 volume in a second or two.
Fluorescence imaging is ideal for detecting multiple probes, each emitting light at a different wavelength, at the same time. This “multiplexed” approach can provide information on both the molecular content and environment of the tissue, and enable endoscopists to rapidly distinguish between normal and precancerous tissues. The Stanford team demonstrated multispectral functionality of the dual-axis microscope by imaging multiple molecular probes that emit in the 500–800 nm range.
Fluorescence imaging enables multiplexing with four or five different fluorophores, but as this number increases it becomes more likely that the signal from one fluorescent dye will overlap with adjacent channels. Ideally, says Contag, a diagnostic system should use nearer to a dozen different channels. To achieve this, the researchers turned their attention to surface-enhanced Raman spectroscopy (SERS) using functionalized SERS nanoparticles as molecular-imaging contrast agents.

These nanoparticles have a layer of Raman-active molecules adsorbed onto a gold core (which acts to significantly enhance the Raman signal) and are coated with a silica layer functionalized with a variety of tumour-targeting agents. By using different Raman-active molecules, each nanoparticle type generates a unique Raman spectral signature, enabling high levels of multiplexing.
To perform in vivo SERS, Contag’s team needed to miniaturize a Raman microscope to create an endoscopic probe. Designing a device for colon-cancer screening, the researchers developed a non-contact, 5.3 mm diameter Raman scanning probe that can scan the entire colon in less than 10 minutes. The probe’s rotating fibre-bundle tip contains an illumination fibre surrounded by 36 collection fibres that direct the Raman signals onto a CCD camera. The spectra are then resolved into individual Raman fingerprints.
For clinical application, the scanning procedure will involve using the endoscope to spray tumour-targeted SERS nanoparticles onto the area of interest during endoscopy. Any unbound particles are washed off prior to scanning and spectral analysis can be performed in real time to determine the presence of any pathological conditions.
In tests on a hollow 5 cm cylindrical phantom (a tissue substitute), the scanning Raman probe could detect particles of different types placed on its inside surface. In further tests on human colon tissue, the device could accurately identify 10 different types of SERS nanoparticle applied to the tissue samples. Furthermore, using known reference spectra, the system was able to separate individual Raman spectra from a mixture of particle types.
The team is also investigating the application of real-time “ratiometric” imaging, in which the relative concentrations of two or more nanoparticle types are determined, with one type serving as a non-targeted control and the other(s) used to target relevant cells. By accounting for any non-specific particle accumulation after washing, as well as for variable working distances, this approach improves the specific detection of bound, targeted nanoparticles. Tests on colon-tissue samples showed detection of picomolar concentrations of SERS nanoparticles, with analytical calculations predicting a detection limit of just 56 nanoparticles per cell.
Contag notes that the nanoparticles have not been tested in humans yet, but says that discussions to do so are ongoing with the US Food and Drug Administration. In the meantime, the team is refining its kit further. For example, his team has already introduced a built-in microsurgical tool – a pulsed electron avalanche knife – that can excise a small cylinder of tissue if disease is detected.
The researchers are also continuing to progress their fluorescence-based dual-axis confocal microscope for applications including visualization of circulating tumour cells in the blood. Other developments include extending optical-imaging approaches to other sites, such as additional hollow organs, accessible organs like the skin and oropharynx, and surgically accessed organs such as the prostate, ovaries, breast or brain.