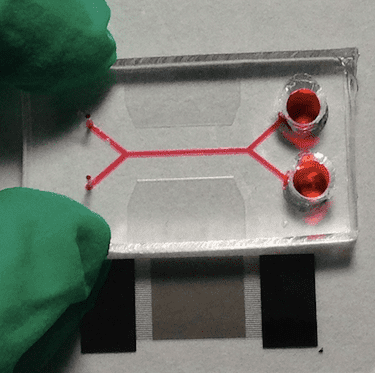
Circulating tumour cells (CTCs) are cancer cells that escape from primary tumour sites and enter the bloodstream. This metastasis is responsible for the majority of deaths from cancer. Monitoring the level of CTC levels in blood is thus important but has proved difficult to do. A team of researchers in China and the US has now developed a new way to isolate these cells using a technique called size-amplified acoustofluidics in which the CTCs selectively bind to microbeads.
The bound cancer cells are significantly different in terms of size and physical properties (they are stiffer, for example) compared to normal cells, explain the researchers led by Feng Guo of Indiana University in Bloomington in the US. This means that their acoustic radiation force is a 100-fold higher than that of bare CTCs or normal blood cells. They can thus be efficiently sorted from blood using microbeads (the “size-amplifiers”) in a travelling acoustic wave microfluidic device, and then released from the amplifiers by being degraded with enzymes.
The technique is 77% efficient and produces CTCs with a 96% yield.
Acoustofluidic devices
Acoustofluidic devices use ultrasonic waves within microfluidic channels to separate suspended micro- and nanoscopic entities (in this case, biological cells). They work thanks to a process called acoustophoresis, in which the suspended objects move when subject to sound energy.
The objects must be smaller in size than the incident wavelength of the sound and the width of the fluidic channels are usually tens to hundreds of microns across. This means that acoustofluidic devices generally make use of ultrasonic waves generated from transducers pulsating at high (megahertz) frequencies. At certain frequencies that depend on the geometry of the devices, it is possible to produce an acoustic field that shifts the moving trajectory of particles across streamlines that flow within the bulk of a fluid. In this procedure, the acoustic radiation force generated by the sound waves is key in sorting the complexes formed between the CTCs and the microbeads, say the researchers, since it is this force that pushes the complexes along in the fluid flow.
Selective capture
In their work, Guo and colleagues used 40-micron diameter silica microbeads coated with biodegradable gelatine and grafted with the capture agent EpCAM. These beads can selectively capture CTCs and can then be removed from them after they have been separated in the acoustofluidic device.
Not only can the microbeads efficiently and specifically bond onto CTCs to form CTC/size-amplifier complexes, they also enhance the differences in physical properties between the CTCs and normal blood cells, as mentioned.
Lab tests
The researchers tested out their platform on 1-millilitre-volume blood samples from eight colorectal patients and eight breast cancer patients. They collected the cell/size-amplifier complex using a centrifuge and then counted the number of cells obtained using an optical microscope. They found that the devices separated several to tens of CTCs respectively. They also calculated how pure the cells were (as the ratio of cells sorted with size-amplifiers versus all cells collected at the device outlet).
The technique could be a promising alternative to patient biopsies that are difficult or impossible to perform in some cases – for example, in some lung or breast cancers. It could also help us better understand the mechanisms involved in cancer metastasis, say Guo and colleagues, and so guide targeted cancer therapy.
The research is detailed in the IOP journal Nano Futures.