Robotic C-arm cone-beam CT (CBCT) provides an indispensable tool for guiding interventional cardiac surgical procedures. CBCT is also valuable within radiotherapy, providing 3D images immediately prior to treatment to ensure precise patient alignment. With any cardiac imaging technique, however, the intrinsic motion of the beating heart causes blurring and image artefacts. And existing motion mitigation approaches based on retrospective gating can create streaking artefacts and deliver unnecessary radiation dose to the patient.
To address these shortcomings, a team at the University of Sydney’s ACRF Image X Institute has developed ACROBEAT (adaptive cardiac cone-beam computed tomography), a gated acquisition protocol that compensates for cardiac motion during the scan. ACROBEAT uses the patient’s electrocardiogram (ECG) signal to adaptively regulate gantry velocity and projection time interval, significantly reducing delivered dose while improving image quality (Phys. Med. Biol. 10.1088/1361-6560/ab03f4).
“Our motivation for developing ACROBEAT was twofold,” explains first author Tess Reynolds. “Firstly, we wanted to improve cardiac imaging within the interventional suite. Being able to acquire 3D images mid-procedure is becoming an invaluable tool for valve replacements, as well as stent and pacemaker placements. Secondly, cardiac motion is an emerging challenge in radiotherapy. Treatment of central lung and mediastinal tumours is difficult due to the proximity to the heart and mediastinum, which are both influenced by cardiac and respiratory motion.”
Single sweep
In retrospectively gated protocols, the gantry performs several rotations at a constant velocity and projection time interval. Only projections acquired when heart motion is minimal are used for image reconstruction, resulting in unnecessary dose to the patient and increasing the risk of cardiac toxicities.
ACROBEAT works differently: the C-arm performs just one sweep of the patient, using the ECG signal to define the angular separation between projections and the projection time intervals in real time. This prospective gating, which uses previous cardiac cycles to help predict future cycles, ensures that projections are only recorded within the defined acquisition window, reducing unnecessary exposure. Optimizing the angular separation between projections, meanwhile, helps improve image quality.

Reynolds and colleagues developed an in silico cardiac imaging model and simulated scans of an XCAT digital phantom using both ACROBEAT and a conventional multi-sweep retrospective ECG gated protocol. The phantom moved according to three patient ECG traces, representing low (average 54 bpm), medium (average 76 bpm) and irregular heart rates.
The team examined acquisition windows of 30%–40% and 60%–70% through the cardiac cycle, representing optimal gating windows with minimal heart motion. They also investigated the 80%–90% window, where large heart motion is observed, and a longer window spanning 60%–80% of the cardiac cycle.
In simulations of the conventional protocol, the C-arm completes six rotations, acquiring a total of 990 projections in a scan time of 42 s. Roughly 10% of these were used for image reconstruction. For ACROBEAT, the researchers performed three simulations for each scenario, acquiring around 100, 125 and 160 evenly spaced projections in scan times of between 25 and 71 s. All projections were used in image reconstruction.
Enhanced images
Cardiac images reconstructed using the conventional protocol contained streak and blurring artefacts for all three ECG traces. In all ACROBEAT simulations, these artefacts almost completely disappeared.
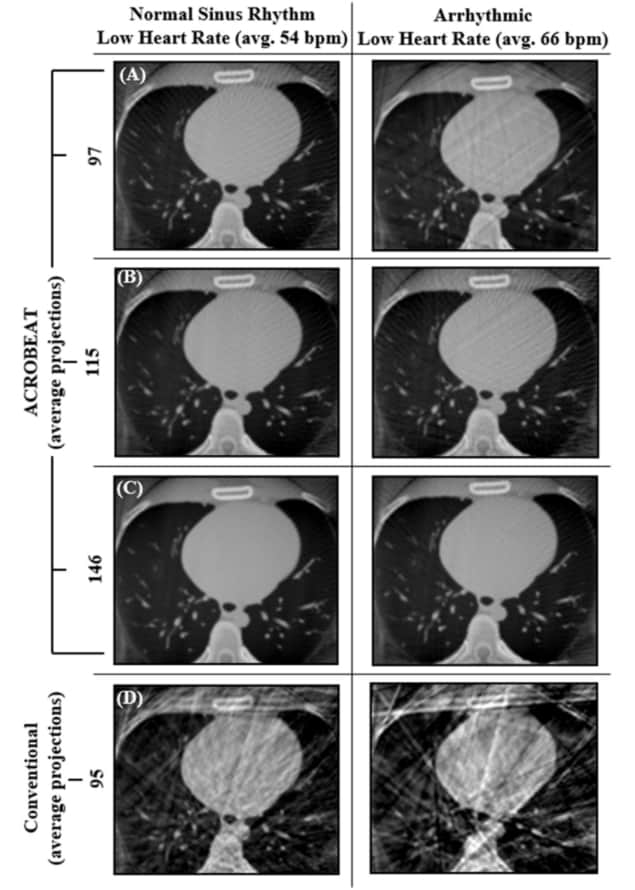
For all ECG traces and 10% acquisition windows, ACROBEAT increased the contrast-to-noise ratio (CNR) compared with the conventional protocol, indicating better visibility of image features. The edge response width (ERW), a measure of boundary sharpness between adjacent regions, was also lower (indicating sharper images) in ACROBEAT simulations than in equivalent conventional images.
Increasing the acquisition window from 60%–70% to 60%–80% resulted in fewer streak artefacts in conventional retrospectively gated images for the two regular ECG traces but not in the arrhythmic trace. Increasing the window length did not have a detrimental effect on either the CNR or ERW of the ACROBEAT images. For medium and arrhythmic heart rates, the 60%–80% window significantly reduced total scan time, although it slightly increased scan time for the low heart rate.
Scan time is important because patients with cardiovascular disease or thoracic cancers typically have reduced breath-hold capabilities. For the 10% acquisition windows, ACROBEAT had an average scan time of 47 s, which is too long for a diseased patient to hold their breath. The extended window, however, decreased the average scan time to 33 s.
“We envisage that a 20% acquisition window would most likely be used,” notes Reynolds. “In its current form, ACROBEAT would still need to be performed under breath-hold conditions to eliminate motion from patient breathing. Therefore, we want to keep the acquisition time as short as possible.”
Overall, ACROBEAT enabled up to a five times average improvement in CNR, a 40% reduction in ERW and an 80% reduction in total projections acquired compared with conventional retrospective ECG gating. The team now plans to undertake the first experimental implementation of ACROBEAT on a robotic C-arm.
“We have an industry partnership with Siemens Healthineers that will provide us with real-time control of a robotic C-arm system, allowing us to test our ACROBEAT protocol,” Reynolds tells Physics World. “In doing so, we are also looking to expand to dual cardiac and respiratory imaging with a robotic C-arm, eliminating the need for a breath-hold.”