Nitric acid and ammonia vapours can condense onto new aerosol particles and rapidly accelerate their growth, the CLOUD experiment (Cosmics Leaving Outdoor Droplets) at CERN has found. This may explain the smog that can engulf megacities on cold winter days, the researchers say, and provides evidence that tighter controls on ammonia emissions from vehicles and other sources are needed.
Winter urban smog occurs when pollution particles form and build up in cold air in and over the city that’s trapped under warmer air at higher altitudes. This temperature inversion suppresses convection, preventing the dispersal of the air pollution. But how these new particles continue to form has been a mystery, as theory suggests that they should be rapidly scavenged by the high concentration of pre-existing particles.
“When there is a winter smog episode, we’re seeing new particles continuously forming and these are making the clouds thicker and more opaque,” explains Jasper Kirkby, head of the CLOUD experiment. “It was not understood how these particles could form, because as soon as a small particle forms within a highly polluted environment it will collide with the pre-existing particles and basically be removed from creating new particles.”
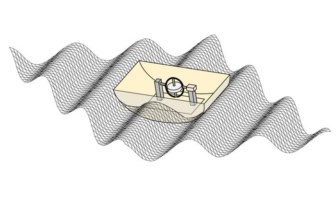
The CLOUD experiment is able to replicate the atmosphere anywhere in the world in a special, ultra-clean chamber. It was designed to investigate the impact of cosmic rays on aerosol, cloud droplet and cloud formation, by taking advantage of CERN’s proton synchrotron, an adjustable source of cosmic rays. But it can be used to explore other atmospheric processes.

In the latest work, published in Nature, Kirkby and colleagues mimicked the range of atmospheric conditions typical of polluted megacities to investigate the role of ammonia and nitric acid in particle formation. They varied the temperature of the chamber from 20°C to −25°C, and adjusted the levels of sulphuric acid, ammonia and nitric acid, as well as aromatic precursors, to cover typical ranges of polluted megacities.
They found that at low temperatures, below about 5°C, nitric acid and ammonia vapours can condense onto freshly nucleated particles that are just a few nanometres in diameter. Due to the high abundance of these vapours, the resulting particle growth rates can be extremely high, reaching well above 100 nanometres per hour. This speeds the particles through the so-called “valley of death”, where very small particles are most vulnerable to loss, in a few minutes.
Kirkby says that nitric acid and ammonia are volatile vapours that are continuously exchanging with particles in the atmosphere, “so they previously were thought to be playing just a passive role, adding a bit of mass to smog, but not really driving the processes”. He adds: “What we found is that they are actually key players and they are helping new particles form in highly polluted environments.”
Due to the strong temperature dependence, the researchers expect the conditions necessary for rapid particle growth to occur in inhomogeneous urban settings, especially in wintertime, driven by vertical mixing and strong local sources of emissions such as traffic. Although the rapid growth may only last for a few minutes at a time, across an urban area this effect could still lead to the build-up of high concentrations of visible particles and dense smog.
The study also found that at temperatures below -15°C, ammonia and nitric acid can condense together and form their own ammonium nitrate particles – which then rapidly grow.
Physicists claim further evidence of link between cosmic rays and cloud formation
Global emissions of ammonia are dominated by farming. In cities, however, ammonia and nitric acid emissions are largely due to vehicles. Vehicle emissions of nitric acid, which derives from nitrogen oxides, are currently controlled. Ammonia emissions, however, are not. In fact, Kirkby says that such emissions are increasing in cities, as catalytic convertors on vehicles are creating ammonia.
According to Kirkby, the study results are significant for human health and urban pollution. “It has highlighted the importance of controlling ammonia inside urban environments,” he explains.