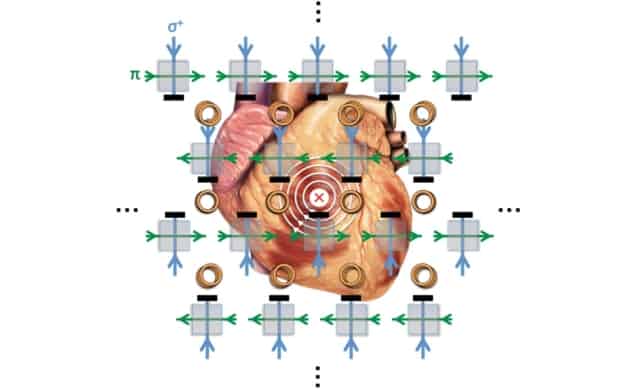
A magnetometer with unprecedented imaging sensitivity promises to bring electromagnetic induction tomography to the clinic. The device, developed by researchers at University College London, images a sample’s conductivity by measuring the magnetic signature of eddy currents induced within it. By demonstrating the technique in a magnetically unshielded environment, the team showed its potential for biomedical applications – for example, to diagnose and guide treatments for atrial fibrillation (Appl. Phys. Lett. 10.1063/5.0002146).
Atrial fibrillation is a life-threatening cardiac arrhythmia in which the electrical pulse that governs the heart’s rhythm is disrupted. The precise causes of the condition are still mysterious, but a leading theory proposes that the disruption is due to anomalies in the electrical conductivity of the heart’s tissue.
“Specifically, clusters of cells in the cardiac muscle become more conductive than they should be,” says Luca Marmugi, who co-authored the research with Cameron Deans and Ferruccio Renzoni. “This creates a sort of ‘short circuit’ in the heart, that prevents the correct propagation of the electrical pulse controlling the heartbeat.”

Monitoring the heart’s naturally generated electrical currents via the magnetic fields that they produce is routine, constituting the well-established field of magnetocardiography. But locating the abnormally conductive cells thought responsible for atrial fibrillation requires a technique that can image the heart’s passive electrical properties as well. Electromagnetic induction imaging (EII) is such a technique.
In EII, a radiofrequency (RF) alternating electric field is used to induce eddy currents in the target under investigation. Measuring the strength of the magnetic field associated with these eddy currents reveals the target’s electrical properties.
But although EII is a proven technique in geophysics and non-destructive testing, biomedical applications have been stymied by the inadequate sensitivity of available magnetometers. To image heart tissue, for example, EII sensors need to detect conductivities of less than 1 siemens per metre (S/m). Even under ideal conditions, atomic magnetometers – the most sensitive instruments developed so far – bottom out at four times that value, and in unshielded, magnetically noisy environments the detection limit rises to 50 S/m.
The device that Marmugi and colleagues used to close this sensitivity gap is based on the same basic instrument employed in those earlier attempts, but with some important refinements. Central to the technique is a vapour cell containing rubidium-87, which the researchers excite into a spin-polarized state with a laser. Meanwhile, the RF field that induces eddy currents in the target simultaneously drives Larmor precession of the Rb atoms’ magnetic moments, making their spin axes wobble like unbalanced spinning tops. The presence of an additional external magnetic field alters the rate and phase of precession, and can be read off in the polarization angle of a linearly polarized laser beam shone into the cell.
In principle, the sensitivity of the baseline version of the device could be improved by boosting the power of the RF field, thereby increasing the induced current and strengthening the resulting magnetic field. In practice, however, raising the RF field strength also broadens the Rb atoms’ spectral lines by a phenomenon called power-broadening – with the overall effect of diminishing the instrument’s sensitivity.
The researchers sidestep this problem using two identical RF coils, which they operate in anti-phase. By placing the vapour cell midway between the two coils, where the fields cancel out, they can avoid power broadening even while ramping up each coil’s individual output. Locating the sample to be imaged nearer to one coil than the other, induces in it strong eddy currents from the increased driving field.
Combined with some other hardware modifications and data-retrieval methods that Marmugi and colleagues have developed over the last few years to handle stray fields, the improved sensitivity of the dual-coil setup enabled them to image a sample with a conductivity of 0.9 S/m. The small size of the sample (5 ml), and the fact that their experiment was unshielded (in a laboratory bathed in the fluctuating magnetic fields from three of London’s main underground train lines) bodes well for the technique’s application to atrial fibrillation.
Next, the researchers intend to perform pre-clinical and clinical studies before rolling out their device to patients.
“In principle, the instrument could be deployed very broadly, from clinical wards to surgical theatres,” says Marmugi. “Thanks to its intrinsic safety, non-invasiveness, and its small power requirements, one could also envisage a more capillary diffusion, potentially – in the longer term – down to GP practices.”