
An automated bone scan algorithm has shown its value in determining the response to radium-223 (Ra-223) treatment for patients with metastatic castration-resistant prostate cancer (mCRPC), according to a study published online October 4 (J. Nucl. Med. 10.2967/jnumed.119.231100).
Swedish researchers compared baseline and post-treatment values from the automated Bone Scan Index (aBSI) software with tumour response biomarkers alkaline phosphatase and prostate-specific antigen (PSA) to see how the trio fared before and after Ra-223 treatment. aBSI was quite helpful in determining how well patients responded to Ra-233 therapy and also could provide their prospects for overall survival.
“In daily clinical practice, there is no validated imaging biomarker for assessment of treatment response,” wrote the authors, led by Aseem Anand from Skåne University Hospital and Lund University. “aBSI has been demonstrated to be a prognostic biomarker in several studies, and, as a fully quantitative assessment of bone, aBSI may have the potential to be a radiographic biomarker to assess efficacious response in metastatic lesions of the bone scan images.”

Prostate metastases
Bone metastases are frequent occurrences in patients with prostate cancer, with estimates as high at 80% that patients with mCRPC will develop skeletal metastases in the spine, skull, ribs and the extremities. The primary modality to detect and assess bone metastases is whole-body bone scintigraphy, which tracks increased uptake of technetium-99m (Tc-99m).
In 2012, Ulmert et al developed the automated Bone Scan Index algorithm to quantify the degree of skeletal tumour burden in bone scans, with calculations based on the percentage of total skeletal weight. The method since has become a “valuable metric and potentially helpful tool for estimating the total quantitative skeletal metastatic burden in patients with mCRPC,” Anand and colleagues wrote. A May 2018 study by Armstrong et al used the aBSI algorithm to predict which prostate cancer patients were more likely to survive after bone scintigraphy with Tc-99m.
“An advantage of aBSI is that it is based on bone scan examinations, which is still the most common method to detect metastatic spread to the bone in patients with prostate cancer,” the Swedish authors wrote.
Relative to patient treatment, radium-223 is designed to selectively target bone metastases and subsequently allow oncologists to plot the most appropriate therapy for these patients. Ra-223 has been shown to extend overall survival by approximately four months with minor or no side effects.
Despite the clinical success of Ra-223 in targeting bone metastases, there has been “little work to evaluate the radiological response in patients being treated with Ra-223,” noted the authors, adding that the “lack of a response biomarker is a significant challenge in clinical routine management of these patients being treated with the Ra-223 treatment.”
Biomarker data
To evaluate the efficacy of aBSI with Ra-223, Anand and colleagues retrospectively gathered bone scan results from seven hospitals in Sweden of 267 patients who were treated with monthly injections of Ra-223 for metastatic castration-resistant prostate cancer. Among the collected data were baseline values of alkaline phosphatase and PSA for 156 patients (median age, 68 years; range, 62–77 years) and for 67 patients (median age, 70 years; range, 64–77 years) within three months of their last Ra-223 injection (median, 5 injections; range, 1–6 injections).
The researchers used aBSI version 3.2 (Exini Diagnostics) to analyse the bone scans and generate each patient’s index at baseline and the end of treatment by segmenting various regions of the skeleton and detecting and classifying the abnormal “hot spots” as metastatic lesions.
Metastatic hot spots
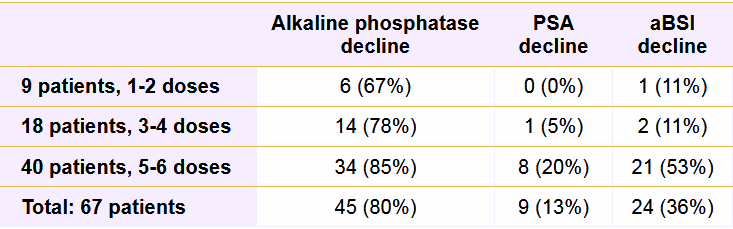
After the last round of Ra-223 treatment, 54 patients (80%) showed a decrease in alkaline phosphatase value, with 38 patients (57%) achieving a decline of 25% or more. By comparison, 24 patients (36%) had a lower aBSI, and nine patients (13%) exhibited a reduction in PSA value.
The number of administered Ra-223 doses correlated with a more advantageous response to treatment. Patients who received five or six doses of Ra-223 were most likely experience declines in alkaline phosphatase (85%), aBSI (53%) and PSA (20%) values. The number of Ra-223 injections also significantly influenced aBSI results, with 21 patients (87%) who received five or six Ra-223 doses having a lower aBSI than three patients (13%) with fewer than five injections.
In the overall survival analysis, baseline aBSI (p = 0.01) and baseline alkaline phosphatase levels (p = 0.001) both significantly correlated with overall survival, while baseline PSA values did not achieve statistical significance (p = 0.60). When combined, the median overall survival of patients with declines in both aBSI and alkaline phosphatase values (median, 134 weeks) also was significantly longer than in patients with only an alkaline phosphatase decline (median, 77 weeks) (p = 0.023).
“For an accurate and comprehensive understanding of disease status, a quantitative and reproducible radiographic assessment like the aBSI can potentially add clinical utility to existing CT evaluation of soft-tissue metastases and to the blood-based biomarkers,” the authors concluded. “In this study, we demonstrated that aBSI at baseline and its change after treatment were significantly associated with [overall survival] and additive to the current predictor of response in mCRPC patients treated with Ra-223. Prospective studies are warranted to validate aBSI as a quantitative imaging response biomarker to Ra-223.”
- This article was originally published on AuntMinnie.com. ©2019 by AuntMinnie.com. Any copying, republication or redistribution of AuntMinnie.com content is expressly prohibited without the prior written consent of AuntMinnie.com.



