3D bioprinting utilizes the mixing of cells with specialized materials to create three-dimensional tissues and organs. These specialized materials are called bioinks and are commonly hydrogel-based to enable the maintenance of cells after printing. A research group from Osaka University in Japan has developed a hydrogel-based bioink that can be gelled using visible light and maintains the pluripotency (ability to differentiate into multiple cell types) of the adipose-derived stem cells (ADSCs) used in this work (Biopolymers doi: 10.1002/bip.23080).
Tailoring the bioink
A common issue with bioprinting is ensuring that the bioink is able to transition from solution to gel quick enough to maintain the printed shape and avoid the 3D construct deforming. In this study, the researchers showed that the hydrogel-based bioink was able to crosslink and gel using visible light. This is an advantage over other types of bioink, which require photo-initiation of crosslinking using ultraviolet light. This approach, which utilizes production of reactive oxygen species to induce crosslinking, also has the potential to cause genetic damage and alter other pathways within the encapsulated cells.
There are multiple different 3D bioprinting procedures available for developing 3D constructs. In this work, the team employed microextrusion, which utilizes an applied force to squeeze out the bioink through the printing head. The specific physical properties of the bioink dictate whether it can form a solid structure without flowing in an uncontrolled manner.
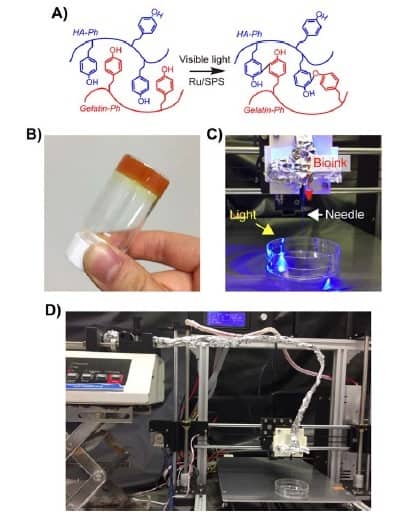
Hydrogels are composed of polymeric chains of peptides, polysaccharides or proteins, which provide structure to a 3D construct when crosslinked. In this study, the bioink utilized chemically modified hyarluronic acid and gelatin to produce a composite hydrogel that can be crosslinked using visible light and allows cells to prosper within the construct.
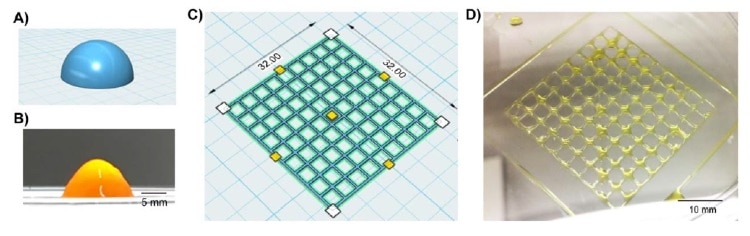
The researchers added a chemical reagent to the bioinks to enable crosslinking of the hydrogel polymers under visible light. This also allowed the group to tune the specific stiffness and gelation rate of the printed bioinks by changing the concentrations of the chemical reagent, or altering the exposure time to the visible light. This tuneability allowed the team to generate 3D-bioprinted constructs for applications based on differing cell and tissue types.
A potential advantage of the use of hyaluronic acid biopolymers is that they have been shown to support maintenance of pluripotency in stem cells in previous studies. This means that the integrity of the stem cells is maintained until factors are introduced to push a stem cell to a chosen lineage. Furthermore, the inclusion of gelatin in the bioink allows for the attachment of cells for growth and migration through a specific peptide domain native to the biopolymer.
Adipose-derived stem cells
The group established the capability of the printed gel to maintain stem cell pluripotency through expression of key pluripotency markers, Nanog, Oct-4 and Sox-2. In addition, the team investigated the differentiation of stem cells to adipocyte cells. Although results indicated that differentiation was successful, they did not complete a full characterization of all markers so the results can only be considered preliminary.
The capability of this bioink to house the cells and maintain their pluripotency implies that the bioink could be useful for future studies with ADSCs. Performance of the bioink in bioprinting of other stem cells would need to be assessed, although the biopolymers used in this study have been used for applications with other stem cells and tissue types. ADSCs are a useful stem cell for research purposes owing to the ease of access of such cells. Arising from fat cells, ADSCs require a simple fat biopsy.
In future, the combination of this bioink with cells of various types could be translated to multiple applications, hopefully resulting in effective tissues and organs for both modelling and transplantation.