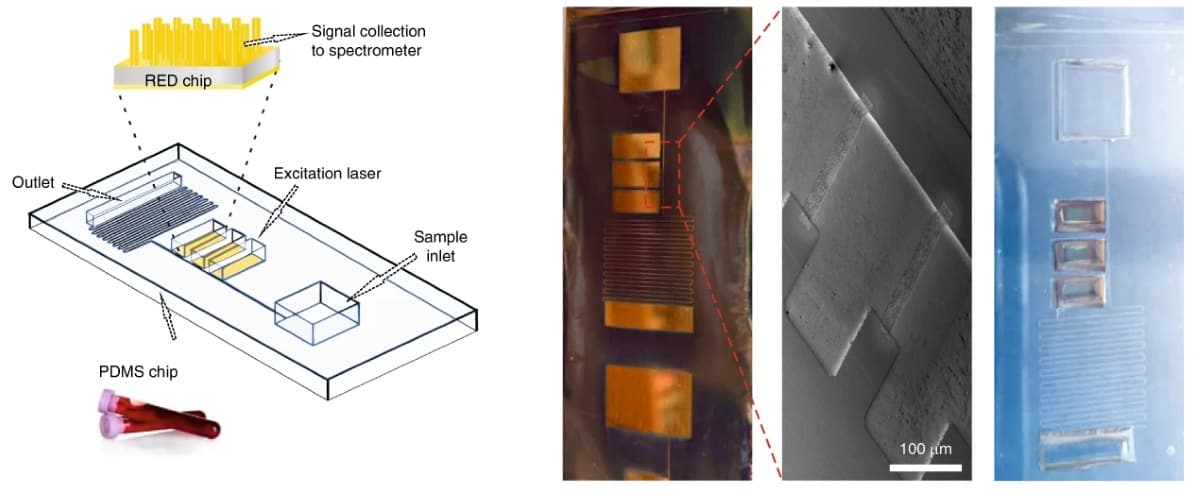
Researchers in the UK claim to have developed a microfluidic chip that can rapidly tell whether someone has suffered a traumatic brain injury from a finger-prick blood sample. The optofluidic device detects a biomarker linked to brain injury, based on the way that it scatters light (Nat. Biomed. Eng. 10.1038/s41551-019-0510-4).
Identifying a traumatic brain injury – where a head injury disrupts normal brain function – isn’t always easy and time is often critical. But with diagnosis often relying on imaging such as CT and MRI scans, assessing all potential cases can be resource intensive.
To address this, Pola Oppenheimer from the University of Birmingham and colleagues developed a lab-on-a-chip system that assesses levels of a molecule produced by the central nervous system after a brain injury. “The idea is to try to develop a portable point-of-care diagnostic assay that allows us to pick up really early stages, really low concentrations of biomarkers indicating traumatic brain injury,” Oppenheimer tells Physics World.
The device rapidly separates plasma from whole human blood through capillary action. The finger-prick blood sample is added to the chip and then flows along a channel and through a series of combs that filter out red blood cells. The separated plasma then flows into an optical detection area.
The plasma sample is analysed using Raman spectroscopy to detect concentrations of N-acetylasparate, one of the most abundant molecules in the central nervous system. The researchers assessed four potential biomarkers, but decided to focus on N-acetylasparate, as evidence suggests it is an effective, specific indicator of traumatic brain injury.
The team tested the setup using blood samples taken as part of a large study looking at early changes in patients who have suffered a traumatic brain injury. The samples came from 35 people with confirmed severe traumatic brain injury, eight people who had suffered head – but not brain – injuries, and 23 healthy volunteers. In the injury groups, the first blood sample was taken by the ambulance crew at the scene of the accident. Additional samples were taken over the next 48 hours. In total, the researchers tested 221 blood samples.
N-acetylasparate levels were on average more than five times higher in patients with traumatic brain injury immediately after injury, than in the other groups. Concentrations were also significantly higher in the brain injury group compared with patients with head injuries only, eight and 48 hours after injury.
The researchers say that the test clearly discriminated between those with traumatic brain injuries and those with other injuries. Overall, they claim that by testing for elevated N-acetylasparate levels, they were able to identify patients with traumatic brain injury with an accuracy of almost 99% immediately after injury, and around 91% at eight and 48 hours after injury.
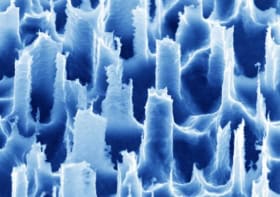
As the concentrations are so low in blood plasma, to analyse N-acetylasparate levels using Raman spectroscopy the team had to enhance the spectral readout. To do this, they performed surface-enhanced Raman spectroscopy using special electrohydrodynamically fabricated surfaces for the detection portion of the chip.
The detection surfaces were created by placing silicon wafers between two electrodes. When a voltage is applied to the electrodes the resulting electrical field and electrostatic forces destabilize the smooth silicon wafer, creating a pattern of pillars. By adjusting the nature of the electrical field, this pattern can be precisely controlled and tailored to enhance the light scattering of specific molecules. Once this process is complete, the wafer is given a fine coating of gold.
“We can control the dimensions really nicely, we can control the height and the width and the spacing between the structures, and this allows us to really carefully tune the different morphologies to match the excitation wavelength and get a really good resonance for high signal enhancement,” Oppenheimer explains.
Ultimately the team hopes to create a device that can be used at accident sites to diagnose and triage patients – to help ambulance crews decide which patients to send to major hospitals with neurosurgical facilities.
Oppenheimer tells Physics World that the next step is a medium-sized clinical trial. But she adds that there could be other healthcare applications: “This is quite a versatile technology, so although we validated it for traumatic brain injury, we are now starting work with other diseases.”