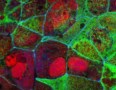
Researchers at the EMBL in Germany have dramatically reduced the time required to create images using Brillouin microscopy, making it possible to study the viscoelastic properties of biological samples far more quickly and with less damage than ever before. Their new technique can image samples with a field of view of roughly 10 000 pixels at a speed of 0.1 Hz – a 1000-fold improvement in speed and throughput compared to standard confocal techniques.
Mechanical properties such as the elasticity and viscosity of biological cells are closely tied to their function. These properties also play critical roles in processes such as embryo and tissue development and can even dictate how diseases such as cancer evolve. Measuring these properties is therefore important, but it is not easy since most existing techniques to do so are invasive and thus inherently disruptive to the systems being imaged.
Non-destructive, label- and contact-free
In recent years, Brillouin microscopy has emerged as a non-destructive, label- and contact-free optical spectroscopy method for probing the viscoelastic properties of biological samples with high resolution in three dimensions. It relies on Brillouin scattering, which occurs when light interacts with the phonons (or collective vibrational modes) that are present in all matter. This interaction produces two additional peaks, known as Stokes and anti-Stokes Brillouin peaks, in the spectrum of the scattered light. The position of these peaks (the Brillouin shift) and their linewidth (the Brillouin width) are related to the elastic and viscous properties, respectively, of the sample.
The downside is that standard Brillouin microscopy approaches analyse just one point in a sample at a time. Because the scattering signal from a single point is weak, imaging speeds are slow, yielding long light exposure times that can damage photosensitive components within biological cells.
“Light sheet” Brillouin imaging
To overcome this problem, EMBL researchers led by Robert Prevedel began exploring ways to speed up the rate at which Brillouin microscopy can acquire two- and three-dimensional images. In the early days of their project, they were only able to visualize one pixel at a time. With typical measurement times of tens to hundreds of milliseconds for a single data point, it therefore took several minutes, or even hours, to obtain two-dimensional images of 50–250 square pixels.
In 2022, however, they succeeded in expanding the field of view to include an entire spatial line — that is, acquiring image data from more than 100 points in parallel. In their latest work, which they describe in Nature Photonics, they extended the technique further to allow them to view roughly 10 000 pixels in parallel over the full plane of a sample. They then used the new approach to study mechanical changes in live zebrafish larvae.
“This advance enables much faster Brillouin imaging, and in terms of microscopy, allows us to perform ‘light sheet’ Brillouin imaging,” says Prevedel. “In short, we are able to ‘under-sample’ the spectral output, which leads to around 1000 fewer individual measurements than normally needed.”
Towards a more widespread use of Brillouin microscopy
Prevedel and colleagues hope their result will lead to more widespread use of Brillouin microscopy, particularly for photosensitive biological samples. “We wanted to speed-up Brillouin imaging to make it a much more useful technique in the life sciences, yet keep overall light dosages low. We succeeded in both aspects,” he tells Physics World.
Brillouin scattering reveals tumour dynamics
Looking ahead, the researchers plan to further optimize the design of their approach and merge it with microscopes that enable more robust and straightforward imaging. “We then want to start applying it to various real-world biological structures and so help shed more light on the role mechanical properties play in biological processes,” Prevedel says.