Clinicians are finally getting their hands on a new generation of image-guided radiotherapy systems that make it possible to visualize the target area during treatment and adapt the radiation dose in real time. Christopher Schultz, who is Professor and Chair of the Radiation Oncology Department at the Medical College of Wisconsin at Froedtert Memorial Lutheran Memorial Hospital (F&MCW), Milwaukee – one of the leading academic centres for cancer care in the US – believes that the technology will “fundamentally transform how radiation therapy regimens are developed, implemented and adapted to achieve optimal outcomes for our patients”.
Schultz and his team are one of the early adopters of Elekta’s Unity system, which combines high-field, 1.5 T magnetic resonance (MR) imaging with a state-of-the-art linear accelerator. Unity was certified for clinical use in Europe in June 2018 and gained US clearance at the end of last year, and the MCW team has been treating patients with the MR-enabled system since January.
According to Schultz, integrating diagnostic quality imaging into the treatment system offers a route to personalized therapy that is tailored to each patient’s tumour and anatomy, and also enables clinicians to adapt treatment in real time as the tumour changes shape and position. “Having image guidance closest to the delivery of the treatment, and compressing the workflow that used to occur over a week or longer into hours, is really powerful,” he comments. “There’s a real opportunity to improve the precision of radiation therapy, and we believe it could offer substantial improvements for certain tumour sites.”
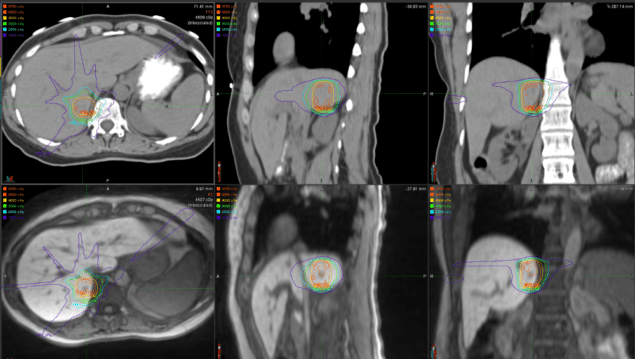
After just a few short months it’s too early for Schultz and his team to properly assess the impact on patient outcomes, but the initial indications are promising. “It’s certainly allowed us to see tumours in the liver, for example, that we wouldn’t have been able to verify otherwise. We’ve also treated an upper abdominal case where we where we were really impressed to see just how much things could move around during treatment.”
Schultz is particularly keen to explore how MR-guided radiotherapy could help to manage the movement of tumours between and during treatments, plus he also sees potential in using adaptive radiotherapy to treat tumours that respond rapidly to treatment, as well as those that prove unresponsive to standard doses of radiation treatment. In this case the ability to define the tumour and its environment make it possible to increase the dose without damaging the adjacent organs and structures. “If you could identify which tumours are responding and which ones aren’t, you may be able to pivot to different treatments on the fly, rather than waiting to complete a whole treatment course,” he says.
Imaging reaches the treatment room
It’s no coincidence that Schultz has been quick to deploy MR-guided radiotherapy in his cancer care clinic. Back in 2007, the F&MCW team started to introduce high-field MR imaging into the treatment planning workflow, and MR physicists were recruited to perfect the technology set-up, MR sequence selection, and planning process – which has been used in around 3000 cases so far. The team has also implemented a computer tomography (CT) imaging and linear accelerator platform for daily repositioning and to capture any changes in the target or surrounding normal structures, which makes it possible to make any daily “online” updates to the treatment plan that may be needed.
“We were very interested in the idea of integrating MR imaging with a linear accelerator, and we had the right skillset with experience of both MR imaging and the CT-based in-room adaptive therapy workflow,” comments Schultz. As a result, the team has been actively involved in bringing the MR linac technology to the clinic, and F&MCW is a founding member of a unique consortium established in 2012 by Elekta and its MR technology partner Philips to ease clinical adoption and share best practice.
The MR-linac Consortium, which initially included six founding members alongside F&MCW, prioritized nine disease sites that would benefit most from the technology. Each of these institutions have since then taken the lead on developing treatments for these different disease sites, with the aim of developing and documenting a common clinical approach that can be adopted by other oncology centres.
The team at F&MCW has a particular interest in pancreatic cancer, in which case the duodenum is often immediately adjacent to the target. “If we can verify that the duodenum is positioned away from the target area in the pancreas, we can be confident in delivering the required radiation dose to the tumour – or even consider increasing the dose on that day,” Schultz explains. “If we can’t avoid the adjacent duodenum, we would just give a conventional treatment or might even cancel the treatment in some circumstances. The ability to account for that day-to-day variability, and then to conform the dose based on those constraints, is a big differentiator for this technology.”
From the initial experiences reported by other consortium members, Schultz says that every disease will offer different opportunities for improving patient outcomes. For the upper abdomen it is particularly important to manage the motion of the tumour and adjacent organs, while in the bladder and the oesophagus it might be possible to reduce the treatment volume as the tumour shrinks – a change that can’t be seen with conventional approaches. In the brain, daily imaging is revealing changes in the amount of swelling around some tumours, which again could provide an opportunity to reduce the size of the treatment target and so safeguard more of the surrounding tissue.
Longer term, says Schulz, the ability of MR imaging to acquire functional or biological images offers a route to targeting the treatment based on biology rather than anatomy. Rather than prescribing a dose based on histology or the stage of a tumour, it might be possible to exploit MR-based imaging biomarkers to determine the dose that would have most impact on the tumour.
“To begin with we are obtaining MR images that show diffusion and perfusion characteristics of a tumour, which are surrogates in part for hypercellularity [the presence of an abnormal excess of cells],” says Schultz. “Even in our first patients where we have obtained daily diffusion imaging, we are seeing changes that, at least at first pass, appear to correspond with reduction in cellularity. That’s pretty interesting.”
Data drives development
The next phase for the consortium – which has now grown to more than 20 members – will be to rigorously report and document patient outcomes from the different clinical approaches. “Whether it’s a question of escalating the dose, reducing the number of treatments, or tailoring the treatment based on the patient’s anatomy, the idea is to capture the outcomes from these nine disease sites,” explains Schultz. “We’re going to learn an awful lot about what really happens by obtaining images every day.”
This, Schultz continues, will generate a repository of outcome data, linked to the imaging data, that will provide a powerful tool for deciding how the technology should be used and for developing new treatment modalities. “By collecting patterns of failure, tumour control, normal tissue toxicity and patient reported outcome data in a consistent and structured way , we will be creating a unique database that will allow us to prove the value of the technology for all stakeholders.”
One of the challenges for any new technology is developing a workflow that works effectively for both the patients and the clinical team, but Schultz says the workflow for the Unity system has been “smoother than anticipated for a brand new system”. In most cases the treatment plan only needs to adapt to changes in the position of the tumour, which only needs a small adjustment to the position of the radiation beam, and the treatment time is limited to around 20–30 minutes. Any change in the shape or size of the target requires some adjustment to the outline of the target or adjacent organs, followed by adaptive re-planning, which extends the treatment time to 40–60 minutes or so.
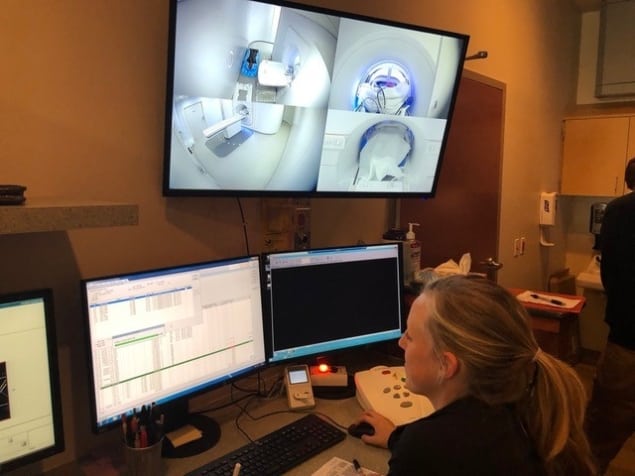
During the treatment, a specialist MR therapist is needed in addition to the standard clinical team, and in these early days a radiation oncologist and medical physicist are also in the room during treatment. Therapists who normally position the patient and deliver the treatment provide the imaging information to the physician, who then decides whether the plan needs to be changed. The physicist is then on hand to launch and verify the replan, and to check the imaging parameters that may affect the radiation dose in the “adapt-to-shape” workflow.
“Having the physicist, radiation oncologist and an extra MR technologist at the machine is an expansion of the typical workflow, but it’s not necessarily that different from the workflow when we use our regular linac with in-room CT imaging,” says Schultz. “We would envision over time that the physicist and physician might not need to be present during treatment, and they could check the output from where they’re sitting.”
Schultz is confident that MR-guided radiotherapy could be introduced at many other oncology centres. “It is really important to have access to MR expertise and to think very methodically about the workflow, but this is not just a research machine that can only be deployed at large medical centres, “ he says. “A number of clinics have already done a lot of the work around the steep part of learning curve, which will make the technology easier and easier to deploy over time.”




