Join the audience for a live webinar at 5 p.m. GMT/1 p.m EST on 18 March 2026
Does electrolyte purity really matter in CO2 electroreduction research? Quite a lot, if you’re doing rotating ring-disk electrode studies. Learn more in this webinar
Want to take part in this webinar?
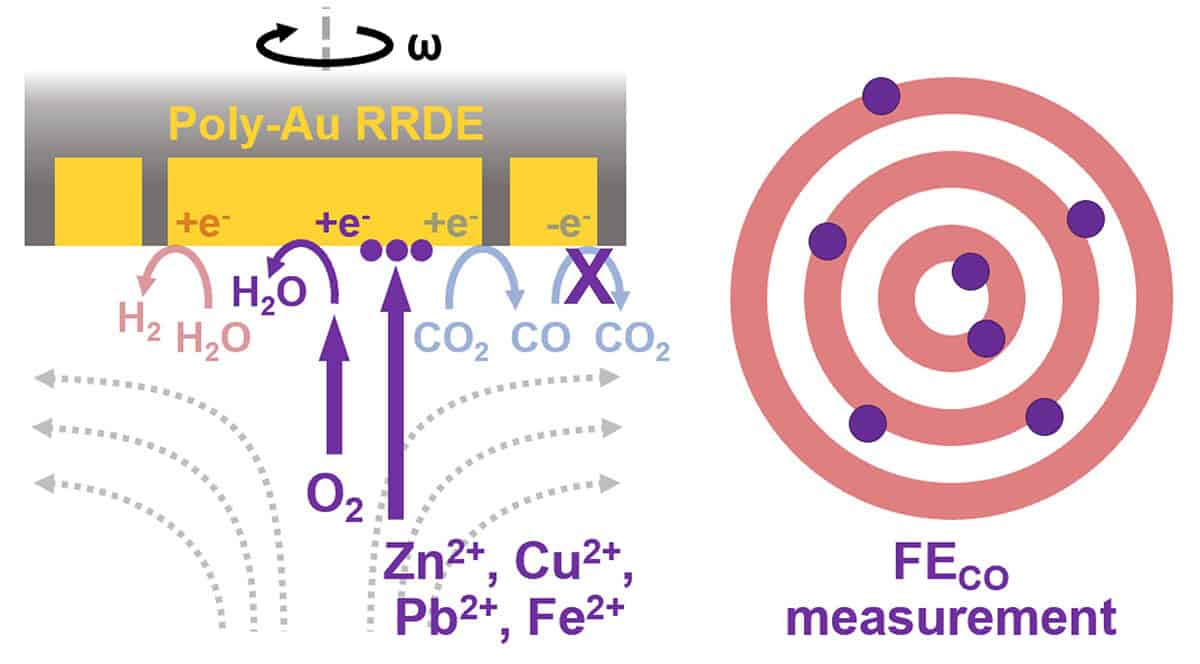
Electrochemical CO2 reduction converts CO2 to higher-value products using an electrocatalyst and could pave the way for electrification of the chemical industry. A key challenge for CO2 reduction is its poor selectivity (faradaic efficiency) due to competition with the hydrogen evolution reaction in aqueous electrolytes. Rotating ring-disk electrode (RRDE) experiments have become a popular method to quantify faradaic efficiencies, especially for gold electrocatalysts. However, such measurements suffer from poor inter-laboratory reproducibility. This work identifies the causes of variability in RRDE selectivity measurements by comparing protocols with different electrochemical methods, reagent purities, and glassware cleaning procedures. Electroplating of electrolyte impurities onto the disk and ring surfaces were identified as major contributors to electrocatalyst deactivation. These results highlight the need for standardized and cross-laboratory validation of CO2RR selectivity measurements using RRDE. Researchers implementing this technique for CO2RR selectivity measurements need to be cognizant of electrode deactivation and its potential impacts on faradaic efficiencies and overall conclusions of their work.
Want to take part in the webinar?

Maria Kelly is a Jill Hruby Postdoctoral Fellow at Sandia National Laboratories. She earned her PhD in Professor Wilson Smith’s research group at the University of Colorado Boulder and the National Renewable Energy Laboratory. Her doctoral work focused on characterization of carbon dioxide conversion interfaces using analytical electrochemical and in situ scanning probe methods. Her research interests broadly encompass advancing experimental measurement techniques to investigate the near-electrode environment during electrochemical reactions.