Researchers from the University of Virginia have published the latest results from the International Diabetes Closed-Loop trial (N. Engl. J. Med. 10.1056/NEJMoa1907863). Their findings suggest that applying a closed-loop approach to the administration of insulin in patients with type 1 diabetes could be a viable alternative to current methods. This could change the life of patients for whom the automatic and efficient monitoring of glucose levels is paramount to control the numerous complications associated with the progress of their condition.
The burden of diabetes worldwide has quadrupled in the last 40 years, particularly in middle- to low-income countries, and is expected to continue to rise in coming years. As a consequence, the incidence of diabetes-related blindness, kidney failure, heart attacks, stroke and lower-limb amputation is likely to keep increasing too. Fortunately, diabetes complications are treatable and can be avoided or delayed by adopting a correct diet, performing physical activity and medicating diabetes-related complications as they appear (according to the World Health Organization).
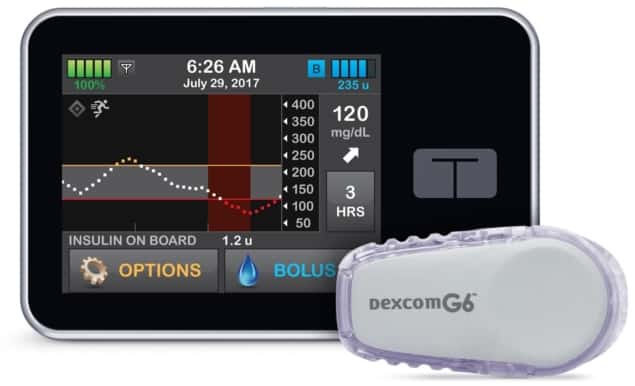
However, despite the constant efforts of physicians and researchers, maintaining a steady glycaemic level in patients affected by diabetes remains elusive. The current gold-standard for diabetes monitoring and treatment consists of constant glucose monitoring and sensor-augmented insulin pumps. These allow the patient to check glycaemia, regulate the basal insulin release from the pump and program the release of additional insulin doses – called boluses – before or after meals.
In this randomized, six-month long study, the researchers assessed the efficiency of a recently commercialized, automated closed-loop system called Control-IQ for the release of insulin. The system integrates live glucose monitoring with an insulin release pump for long-term modulation of glucose level.
More efficient than existing devices
The trial showed highly encouraging results. The researchers compared the length of time that the novel device kept patients’ glucose levels within a safe range (70 to 180 mg/dl) with that of another commonly used insulin pump. They estimated that patients receiving insulin through the new closed-loop system benefited from an average of 2.6 hours longer normal glucose levels per day than the control group.
The study also examined a plethora of secondary outcomes, including mean glucose level, glycated haemoglobin, time spent in hypoglycaemia (glucose levels below 70 mg/dl) or severe hypoglycaemia (below 54 mg/dl), and time spent in hyperglycaemia (above180 mg/dl). Importantly, the closed-loop system was superior to the insulin pump in all of these secondary outcomes, too.
On the other hand, the tested closed-loop device showed an increased number of adverse events. Patients using this system were significantly more subject to hyperglycaemia or ketosis events (without diabetic ketoacidosis) than the control group. The team attributed these defects to failures in the infusion set, which could reflect the difference in requirements for adverse event reporting between the two groups, as the insulin pump in the closed-loop system was part of an investigational device.
Strength in numbers
One strength of this study was the large number of participants, representing a wide range of variables: they were aged from 14 to 71 years old; had had diabetes for one to 62 years; and their baseline glycated haemoglobin ranged from 5.4 to 10.6%. The authors concluded that the tested closed-loop device led to a significant improvement of glycaemia levels and glycated haemoglobin during the six-month trial, suggesting that closed-loop systems may provide an interesting option to consider when deciding on a patient’s diabetes treatment.



