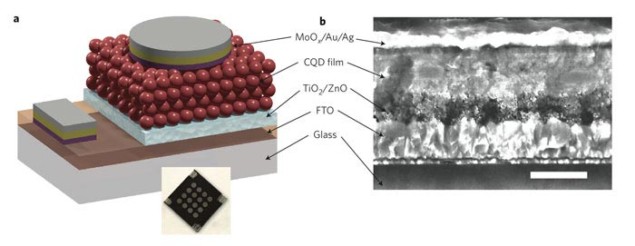
Researchers at the University of Toronto in Canada and KAUST in Saudi Arabia have made a solar cell out of colloidal quantum dot (CQD) films that has a record-breaking efficiency of 7%. This is almost 40% more efficient than the best previous devices based on CQDs.
CQDs are semiconductor particles only a few nanometres in size. They can be synthesized in solution, which means that films of the particles can be deposited quickly and without fuss on a range of flexible or rigid substrates – just as paint or ink can be.
CQDs could be used as the light-absorbing component in cheap, highly efficient inorganic solar cells. In a solar cell, high-energy photons hitting the photovoltaic material can produce excited electrons and holes (charge carriers) that have energies at least equal to or greater than the band gaps of the material. The advantage of using CQDs as the photovoltaic material is that they absorb light over a spectrum of wavelengths. This is possible because the band gap of a CQD can be tuned over a large energy range by simply changing the size of the nanoparticles.
Trapped electrons
There is a snag, however – the high surface-area-to-volume ratio of nanoparticles results in bare surfaces that can became “traps” in which electrons invariably get stuck. This means that electrons and holes have time to recombine instead of being whisked apart to produce useful current. The result is a reduction in the efficiency of devices made from CQD films. A team led by Edward Sargent at Toronto may now have come up with a solution to this annoying problem. The researchers have succeeded in passivating the surface of CQD films by completely covering all exposed surfaces using a chlorine solution that they added to the quantum-dot solution immediately after it was synthesized. “We employed chlorine atoms because they are small enough to penetrate all of the nooks and crannies previously responsible for the poor surface quality of the CQD films,” explains Sargent.
The team then spin cast the CQD solution onto a glass substrate that was covered with a transparent conductor. Next, an organic linker was used to bind the quantum dots together. This final step in the process results in a very dense film of nanoparticles that absorbs a much greater amount of sunlight.
Boosting absorption
“Our hybrid passivation scheme employs chlorine atoms to reduce the number of traps for electrons associated with poor CQD film-surface quality while simultaneously ensuring that the films are dense and highly absorbing thanks to the organic linkers,” Sargent says.
Electronic-spectroscopy measurements confirmed that the films contained hardly any electron traps at all, he adds. Synchrotron X-ray scattering measurements at sub-nanometre resolution performed by the scientists at KAUST corroborated the fact that the films were highly dense and contained closely packed nanoparticles. “Most solar cells on the market today are made of heavy crystalline materials,” explains Sargent, “but our work shows that light and versatile materials such as CQDs could potentially become cost-competitive with these traditional technologies. Our results also pave the way for low-cost photovoltaics that could be fabricated on flexible substrates, for example using roll-to-roll manufacturing (in the same way that newspapers are printed in mass quantities).”
Exploring new materials
The team is now looking at further reducing electron traps in CQD films for even higher efficiency. “It turns out that there are many organic and inorganic materials out there that might well be used in such hybrid passivation schemes,” adds Sargent, “so finding out how to reduce electron traps to a minimum would be good.”
The researchers say that they are also interested in using layers of different-sized quantum dots to make a multi-junction solar cell that could absorb over an even broader range of light wavelengths.
The research is described in Nature Nanotechnology.



