It’s practically a law that no experiment ever works better than theory says it should, but that’s exactly what happened in atomic physics in the late 1980s, as Chad Orzel describes in the second instalment of his three-part history of laser cooling. The first part can be read here
In the late 1960s a small community of researchers began using forces from light to push small objects around. Within the next decade, the field expanded to include laser cooling, a powerful technique that exploits the Doppler shift to produce a force that can only slow objects down, and never speed them up. As the years went by, these new laser cooling experiments developed along the two parallel tracks – ions and atoms – explored in part 1 of this series: “Cold: how physicists learned to manipulate and move particles with laser cooling”.
In many ways, ions had an early advantage. Due to their electric charge, they experience electromagnetic forces, which are strong enough to allow them to be caught in electromagnetic traps at high temperatures and cooled by lasers at ultraviolet wavelengths. By 1981 ion trappers had refined this technique to the point where they could trap and detect single ions and perform spectroscopy on them with unprecedented precision.
Atoms, in contrast, need to be slowed down before they can be trapped by weaker forces exerted by light and magnetic fields. Still, by 1985 Bill Phillips and colleagues at the US National Bureau of Standards in Gaithersburg, Maryland, had used light to slow a beam of sodium atoms almost to a halt, then confined them in a magnetic trap. Beyond that, the principal challenge for would-be atom tamers seemed to involve building on this work to make trapping of neutral atoms more efficient, and pushing the limits of the cooling process itself.
Both projects would succeed beyond anyone’s expectations. And just as we saw in part 1, the roots of this success go back to Arthur Ashkin at Bell Labs.
Good idea, inadequate execution
When we last met Ashkin, it was 1970 and he had just developed the “optical tweezing” technique that would win him a Nobel Prize nearly 50 years later. By the end of the 1970s he was working with his Bell Labs colleagues on experiments involving an atomic beam. “Rick Freeman had an atomic beam machine, and I had some experiments that would be interesting to do with an atomic beam, but I wasn’t too enthused about building an atomic beam machine,” recalls Ashkin’s then-colleague John Bjorkholm.
By overlapping a laser beam with the beam of atoms, Ashkin and Bjorkholm showed it was possible to focus or de-focus the atoms by adjusting the frequency of the light. With the laser tuned to the red – at a slightly lower frequency than the atoms “want” to absorb – the interaction between atoms and light would lower the atoms’ internal energy (the “light shift”), drawing atoms into the laser beam. With the laser tuned to the blue, the atoms got pushed out.
Ashkin had several ideas for turning this phenomenon into an “all-optical” method for trapping atoms (that is, without the magnetic fields Phillips’ group used). Unfortunately, Ashkin and Bjorkholm struggled to implement it because Freeman’s atomic beam was built with plexiglass windows that couldn’t sustain low enough pressures. The atoms and molecules that leaked in from the outside were not affected by the cooling lasers, and as a result, when they collided with atoms in the beam, they kicked the target atoms out of the trap. After a few years of disappointing results, the Bell Labs leadership soured on the experiments and pushed Ashkin to pursue other things.
Swimmers in a viscous fluid

Around this time, a young researcher with a (self-described) reputation as “a guy who could get difficult experiments done” moved into an office near Ashkin’s in Bell Labs’ Holmdel facility. His name was Steve Chu, and he became interested in Ashkin’s ideas. Together, they built an ultrahigh vacuum system suitable for atom cooling and trapping, plus a system to slow sodium atoms by rapidly sweeping the laser frequency to compensate for the changing Doppler shift. The latter technique is known as “chirp cooling”; by happy coincidence, the scientists who developed one of its key technologies were also at Holmdel.
At this point, Chu suggested they pre-cool the atoms by illuminating them with three perpendicular pairs of counter-propagating laser beams, all tuned to a frequency just below the atoms’ transition frequency as discussed in part 1. This configuration provides a cooling force in all three dimensions simultaneously: an atom moving up sees the downward-going laser beam Doppler shifted up, absorbs photons, and slows down; an atom moving left sees photons in the rightward-going beam shifted up, and so on. No matter which way the atoms move, they feel a force opposing their motion. The similarity to the plight of a swimmer in a viscous fluid led Chu to dub it “optical molasses” (figure 1).
1 Optical molasses

An atom is illuminated by pairs of red-detuned beams along perpendicular axes. A leftward-moving atom will see the rightward-going laser Doppler shifted up, and be more likely to absorb light from it, and slow down; the other beams are not shifted, and thus not absorbed. If the atom moves up, it will see only the downward-going beam shifted up, and absorb from it, and so on. The atom experiences a force slowing it down no matter what direction it moves.
The Bell Labs team demonstrated optical molasses in 1985, collecting thousands of atoms from a chirp-cooled beam. As befits the name, the optical molasses was very “sticky”, holding atoms in the overlapping beams for around a tenth of a second (practically an eternity in atomic physics) before they wandered out. While in the molasses region, the atoms are constantly absorbing and re-emitting light from the cooling lasers, so they appear as a diffuse glowing cloud. The total amount of light provided an easy measure of the number of atoms.
Ashkin, Chu and their collaborators were also able to estimate the atoms’ temperature. They did this by measuring how many atoms were in the molasses, switching off the light for a short time, then turning it back on and re-measuring the number. During the dark interval the atom cloud would expand, and some atoms would escape the region of the molasses beams. This escape rate allowed the team to calculate the atoms’ temperature: about 240 microkelvin – right in line with the expected minimum for laser-cooled sodium atoms.
Turning molasses into a trap
Despite its stickiness, optical molasses is not a trap. Although it slows atoms down, once the atoms drift to the edge of the laser beams, they can escape. A trap, in contrast, supplies a force that depends on position, pushing atoms back into a central region.
The simplest way to create a trap is with a tightly focused laser beam, similar to the optical tweezers Ashkin developed for trapping microscopic objects. While the volume of the laser focus is a tiny fraction of the molasses volume, Ashkin, Bjorkholm and (independently) Chu realized that a significant number of atoms could nevertheless accumulate in such a trap through random diffusion in the molasses. When they added a separate, trapping laser beam to their molasses, the results were promising: a small bright spot appeared in the diffuse molasses cloud, representing several hundred trapped atoms.
Getting beyond that, however, presented technical challenges. Trouble is, the shift in atomic energy levels that makes single-beam optical trapping possible hampers the cooling process: when the trapping laser lowers the energy of the atom’s ground state, it changes the effective frequency detuning of the cooling laser. Using a second laser and alternating between cooling and trapping improves the number of atoms that can be trapped, but at the cost of additional complexity. To make further progress, physicists would need either colder atoms or a better trap.
The French connection

Both were on the horizon. Claude Cohen-Tannoudji and his group at the École Normale Supérieure (ENS) in Paris were primarily addressing laser cooling from the theoretical side. Jean Dalibard, then a newly minted PhD in the group, remembers studying theoretical analyses by Ashkin and Jim Gordon (“a fantastic paper”) and by the Soviet duo of Vladilen Letokhov and Vladimir Minogin, who (with Boris D Pavlik) had derived the minimum temperature achievable with laser cooling back in 1977.
As we saw in part 1, this minimum temperature is known as the Doppler cooling limit, and it stems from the random “kicks” that occur when atoms re-emit photons after absorbing light from one of the cooling beams. Curious about how firm this “limit” really was, Dalibard looked for ways to keep the atoms “in the dark” as much as possible. To do this, he exploited a property of real atoms that is not captured by standard Doppler cooling theory: real atomic states are not single energy levels, but collections of sublevels with the same energy but different angular momenta (figure 2).
These different sublevels, or momentum states, change energy in the presence of a magnetic field (the Zeeman effect). As the field gets stronger, some states increase in energy, while others decrease. These roles are then flipped when the direction of the field reverses. A further complicating factor is that the polarization of the laser light determines which sublevels will absorb photons. While one polarization moves atoms between states in a way that increases angular momentum, another decreases it.
2 Multiple sublevels in sodium

In the absence of a magnetic field, the ground state of the sodium atom has five sublevels with the same energy but different angular momentum, and the excited state has seven. All transitions between ground and excited state involve light of the same frequency. When a magnetic field is applied, the sublevels shift up or down by different amounts. As a result, the transition between the “stretched state” sublevels of maximum angular momentum move to higher (blue) or lower (red) frequency.
In his theoretical analysis, Dalibard combined these sublevels with a magnetic field that is zero at some point and increases as atoms move outward. In doing so, he created a situation where the effective laser frequency detuning depended on the atoms’ position. (Phillips and colleagues used a similar configuration for their magnetic trap, but at a much higher field.) Atoms could therefore absorb from a particular laser only at the specific position where the combination of detuning, Doppler shift, and Zeeman shift were just right (figure 3).
3 Magneto-optical trap
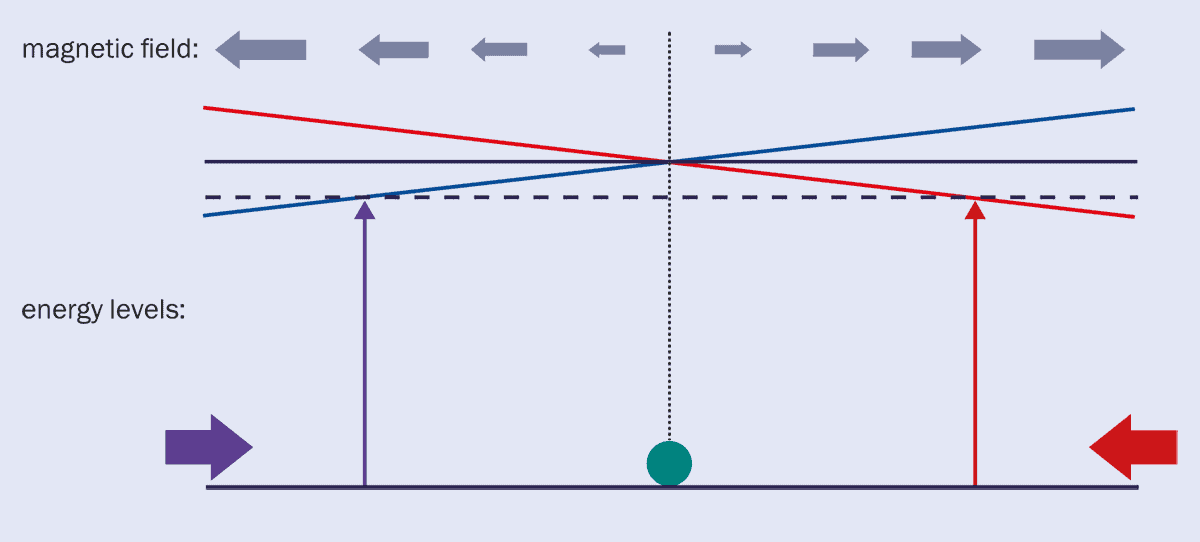
Atoms are illuminated by a pair of red-detuned lasers with opposite polarizations, in a magnetic field that increases moving out from the centre. The sublevels of the excited state shift in opposite directions due to the field, and atoms absorb light only at the position where the combination of detuning, Zeeman shift, and Doppler shift are just right, pushing them back to the centre.
Dalibard hoped that restricting the atoms’ ability to absorb light in this way might lower their minimum temperature. After he calculated that it wouldn’t, he filed the idea away. “I saw it was a trap, but I was not looking for a trap, I was looking for sub-Doppler cooling,” he explains.
That might have been where it ended if it hadn’t been for Dave Pritchard, a physicist at the Massachusetts Institute of Technology who visited the Paris group in 1986. During the visit, Pritchard gave a talk on ideas for producing larger-volume traps, and finished up by saying he would welcome other – better – suggestions.
“I went to Dave, and I said ‘Well, I have an idea, and I’m not too sure it is better, but it is different than yours,’” Dalibard recalls. Pritchard took Dalibard’s idea back to the US, and in 1987 he and Chu built the first magneto-optical trap (MOT) based on Dalibard’s analysis. Dalibard was offered co-authorship of the resulting paper but was happy simply being recognized in the acknowledgements.
It is hard to overstate how revolutionary the MOT was for the development of laser cooling. It’s a relatively simple device, requiring only a single laser frequency and a relatively weak magnetic field to produce strong traps. Best of all, though, is its capacity. Chu and Ashkin’s first all-optical trap held hundreds of atoms, Phillips’s first magnetic trap several thousand, but the first magneto-optical trap held ten million atoms. Together with the introduction of cheap diode lasers by Carl Wieman at the University of Colorado (about which more in part 3 of this series), the advent of the MOT triggered a rapid explosion in the number of groups studying laser cooling worldwide. The pace of research was about to accelerate.
Murphy’s law takes a holiday
While Pritchard and Chu were building the first MOT, Phillips and his Gaithersburg colleagues were encountering an extremely unusual problem with their optical molasses. Contrary to every expectation of experimental physics, the molasses worked too well. In fact, it could cool atoms even with some of its beams partially blocked.
This discovery came about in part because laser cooling was supposed to be Phillips’ side project, so his lab was set up in a prep room connected to a machine shop. To prevent shop dust and grease from accumulating on the lab’s vacuum system, members of the group would cover the system’s windows with plastic or filter paper at night. “Occasionally you would get this really distorted looking molasses,” recalls Paul Lett, who joined the group in 1986, “and then you’d realize that, oh, we didn’t take that piece of filter paper out. It was remarkable that it worked at all.”
This surprising persistence led Lett to push for a more systematic study, including a new set of temperature measurements. The “release-and-recapture” method developed by the Bell Labs group had relatively large uncertainties, so Phillips’ group tried a new method that involved detecting the light emitted as atoms crossed a probe beam placed near the molasses. When the molasses was turned off, the atoms would fly away. The time they took to reach the probe would give a direct measure of their velocity, and thus their temperature.
Like all laser cooling experiments, Phillips’ lab packed a lot of lenses and mirrors into a tiny space, and the most convenient place to put the probe turned out to be slightly above the molasses region. This should have worked fine for atoms travelling at their Doppler-limit speed, but when Lett tried the experiment, no atoms reached the probe. Eventually, he and his colleagues shifted the probe’s position to below the molasses, at which point they saw a beautiful signal. There was just one problem: the Doppler cooling limit was 240 microkelvin, but this “time-of-flight” measurement showed a temperature of 40 microkelvin.

This result seems to violate Murphy’s law, the dictum that “anything that can go wrong, will”, so they weren’t willing to accept it immediately. They re-measured the temperature using several different techniques, including an improved release-and-recapture, but they kept getting the same result: the atoms were much colder than the theory said was possible.
Early in 1988 Phillips and company reached out to other groups in the close-knit community of laser coolers, asking them to check the temperatures in their own labs. Chu and Wieman quickly confirmed the surprising result: optical molasses not only worked to cool atoms, it worked better than theory said it would.
Climbing up a hill
The Paris group did not yet have an experimental programme, but Dalibard and Cohen-Tannoudji attacked the problem theoretically via the same real-world factor Dalibard used to develop the MOT: multiple internal atomic states. The ground state of sodium has five sublevels with the same energy, and the distribution of atoms among those states depends on the intensity and polarization of the light. This distribution process, called “optical pumping,” was central to the spectroscopic research taking place at the ENS in Paris under Cohen-Tannoudji, so his group was uniquely well-suited to exploring how these additional states could improve laser cooling.
The key feature turns out to be the polarization of the laser light, which in classical physics corresponds to the axis of the light’s oscillating electric field. The combination of six counter-propagating beams produces a complicated distribution of polarizations as the beams combine in different ways in different places within the optical molasses. The atoms are constantly being optically pumped into different configurations, extending the cooling process and allowing lower temperatures.
By the summer of 1988 Dalibard and Cohen-Tannoudji had devised an elegant model to explain sub-Doppler cooling. (Chu independently arrived at a similar result, which he recalls deriving on a train between two conferences in Europe.) They considered a simplified atom with only two ground state sublevels, traditionally labelled –½ and +½, illuminated by two laser beams propagating in opposite directions with opposite linear polarizations. This creates a pattern that alternates between two polarization states, labelled σ– and σ+.
An atom in a region of σ– polarization will be optically pumped into the –½ state, which experiences a large light shift that lowers its internal energy. As the atom moves toward the σ+ polarization region, the light shift decreases, and the atom must slow down to compensate, losing kinetic energy to compensate for the increase in internal energy, like a ball rolling up a hill. When it reaches the σ+ light, optical pumping will cause it to switch to the +½ state, which has a large light shift. The atom doesn’t get back the energy it lost climbing the “hill” out of the σ– region, though, so it’s moving slower as the process starts over: the light shift decreases as it moves toward the next σ– region, so it loses energy, then optically pumps to –½, and so on.
This process of losing energy by constantly climbing “hills” supplied a vivid name: Dalibard and Cohen-Tannoudji dubbed it Sisyphus cooling, after the king in Greek myth who was condemned to spend eternity pushing a boulder up a hill only to have the rock slip away and return to the bottom (figure 4). Atoms in optical molasses find themselves in a similar predicament, always climbing hills and losing energy only to have optical pumping return them to the bottom and force them to start over again.
4 Sisyphus cooling

A moving atom in the –½ state sees a large light shift lowering its internal energy when bathed in light with sigma-minus polarization. As it moves towards a region containing sigma-plus polarized light (red area of the diagram), the light shift decreases and the atom slows down to make up for the change in energy. When it gets to the σ+ region, optical pumping moves it to the +½ state where its internal energy is low, but it is still moving slower. Then the process repeats: moving toward σ–, slowing down, optically pumping to –½, etc.
The rewards of Sisyphus
The theory behind Sisyphus cooling makes concrete predictions about minimum temperatures and how they depend on the laser detuning and magnetic field. These predictions were quickly confirmed in labs around the world. In autumn 1989 the Journal of the Optical Society of America B published a special issue on laser cooling containing experimental results from Phillips’ group at Gaithersburg, the Sisyphus theory from Paris, and a combined experimental and theoretical paper from Chu’s group, which had by then moved from Bell Labs to Stanford University in California. For most of the next decade, this special issue was regarded as the definitive source for students seeking to understand laser cooling, and Cohen-Tannoudji and Chu went on to share the 1997 Nobel Prize for Physics with Phillips.
Taken to its limit, the Sisyphus effect can cool atoms to the point where they no longer have enough energy to climb even a single “hill” and are instead confined to a tiny region of a single polarization. This confinement is as tight as it is for trapped ions, making the two branches of laser cooling nicely symmetric. By the early 1990s trapped ions and neutral atoms could both be cooled to a regime where their quantum natures become apparent: a single ion in a trap, or an atom in a “well” created in Sisyphus cooling, can only exist in certain discrete energy states. These discrete states were soon measured for both systems; today, they are an essential part of quantum computing with atoms and ions.
A further intriguing avenue of research concerned the wells themselves. These are formed when light beams interfere, and naturally occur in large arrays with a spacing of half the laser wavelength. The periodic nature of these so-called optical lattices mimics the microscopic structure of solid matter, with the atoms playing the role of electrons in a crystal lattice. This similarity makes trapped atoms a useful platform for exploring condensed-matter physics phenomena such as superconductivity.
To really explore superconductivity with cold atoms, though, the lattice must be loaded with atoms at a higher density and an even lower temperature than can be achieved with Sisyphus cooling. As we’ll see in part 3, getting there would require yet another new set of tools and techniques, and would open the possibility of creating not just analogues of known systems, but entirely new states of matter.