A binary colloidal mixture can exist in up to six phases at the same time, according to new theoretical work by researchers in the Netherlands and France. The result, obtained using a simple algebraic model, has applications for predicting the phase stability of common colloidal materials such as paint and mayonnaise.
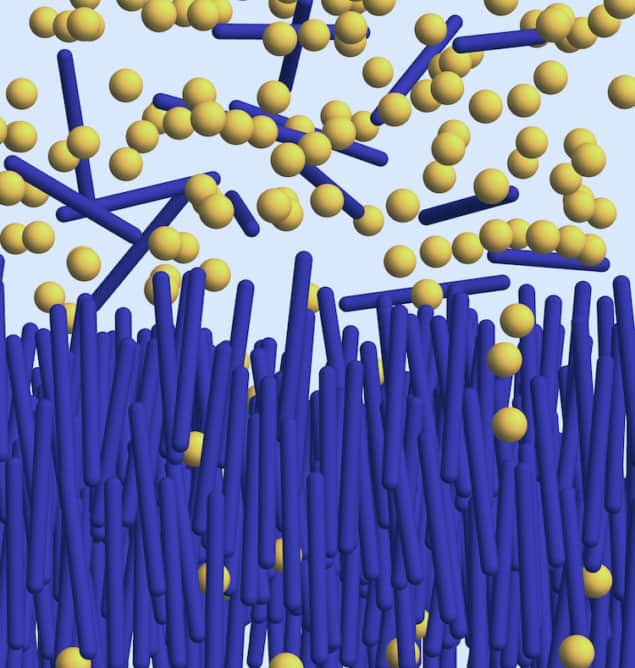
Until recently, scientists thought that colloidal-polymer mixtures could only exist in three simultaneous phases – similar to the well-known “triple point” at which water is a gas, a liquid and a solid. In 2020, however, members of this same Netherlands-France group, led by Remco Tuinier and Joeri Opdam of the Eindhoven University of Technology, showed that such mixtures could, in fact, exist in five different phases at once. This result defied the 150-year-old Gibbs phase rule, and the researchers obtained it by simulating the behaviour of a colloidal mixture with two components – rods and polymers – dispersed in a background solvent.
In their computations, they represented rods as hard “spherocylinders” and the polymers as spheres that freely overlap with each other. They found that in this system, under specific conditions, a “quintuple point” appears in which the possible phases are an isotropic gas phase with unaligned rods at the top; a nematic liquid crystal phase with rods pointing in roughly the same direction; a smectic liquid crystal phase with rods lying in different layers; and two solid phases with “ordinary” crystals at the bottom.
A wide range of possible coexisting multiphases
The same team has now developed a general way to predict phase diagrams for complex mixtures containing two types of hard particles. They did this using an extension of their previous technique that takes into account not only binary mixtures of rods and polymers, but also binary mixtures of, for example, rods and platelets (discs), or mixtures of spheres and rods. The team also considered the orientation and positions of each component in the mixture.
As before, Tuinier, Opdam and colleagues found a wide range of possible coexisting multiphases, including two quintuple phase coexistence regions for rod/sphere mixtures and even a sextuple phase coexistence region for rod/plate mixtures.
This profusion of phases is possible because of the shape of the particles (particularly their length and diameter), they say. In addition to the known variables of temperature and pressure, there are three additional parameters: for both particles, the length in relation to its diameter; and the diameter of one type of particle in relation to the diameter of the other particles in the solution. Thanks to these three parameters, three additional coexisting phases can be possible with respect to the predictions of Gibbs’ phase rule.
Applications in manufacturing
Tuinier notes that being able to predict phase diagrams is important in several areas of science and engineering, where it helps to know when systems will be stable, whether demixing occurs and what kinds of phase states are expected to form. According to the researchers, who report their work in J. Phys. Chem. Lett., the findings could help advance our understanding of phase transitions in such systems and predict more precisely when they will occur – knowledge that could come in useful when manufacturing complex colloidal mixtures like mayonnaise or paint.
‘Quintuple point’ material defies 150-year-old thermodynamics rule
“We wanted to better predict the phase stability of such systems,” Tuinier tells Physics World. “While our new work still focuses on hard particle dispersion of different shapes, we hope to extend this work to also include materials like polymers for practical applications.”
He adds that mixing colloids with different sizes and shapes and employing phase transitions may pave the way towards creating novel materials with bespoke optical or mechanical properties for applications such as fast-switching optical devices or biomimetic muscles. Liquid crystals in displays, photovoltaic and energy storage materials could benefit too, as could novel types of batteries and fuel cells.




