The UK’s Clatterbridge Cancer Centre is realizing sustained efficiencies across its patient and machine QA workflows thanks to an exclusive technology partnership with Sun Nuclear Corporation

Continuous improvement, patient safety, automation and workflow efficiency: these are the operational reference points that underpin a comprehensive portfolio of clinical services at The Clatterbridge Cancer Centre NHS Foundation Trust, one of the UK’s leading oncology programmes delivering non-surgical cancer care – radiation therapy, chemotherapy, immunotherapy and gene therapy – to approximately 30,000 patients every year in Liverpool and the wider metropolitan region across the north-west of England.
For its part, the Trust’s 40-strong medical physics team has put those reference points front-and-centre over the past five years, having successfully phased in a unified suite of 11 linacs from Varian Medical Systems across the Clatterbridge’s three specialist radiotherapy clinics. The resulting treatment systems comprise a mix of Edge, TrueBeam and Clinac iX machines, all of them using plans from Varian’s Eclipse treatment planning system.
Convergent thinking
That strategic move to consolidate and standardize the radiotherapy workflow was reinforced when the Clatterbridge team subsequently implemented a single-source radiotherapy QA programme in partnership with Sun Nuclear Corporation, the US-based manufacturer of independent QA solutions for radiotherapy facilities and diagnostic imaging providers. Put simply, the shared goal here is to support a unified framework for treatment planning, management and delivery by driving QA automation and best practice across the Clatterbridge radiotherapy programme.
“It’s fair to say that previously we had an overly complex QA environment,” explains Greg Martin, a radiotherapy physicist based at Clatterbridge Cancer Centre-Liverpool, the latest addition to the Trust’s network of clinics. “We’d created a whole new industry for ourselves, with technology fragmentation resulting in a significant training burden and maintenance overhead as a result of QA hardware and software tools being sourced from multiple equipment vendors.”
Conversely, the establishment and evolving implementation of Sun Nuclear’s SunCHECK Quality Management Platform – a single interface and database that provides a unified view of patient and machine QA – has enabled Clatterbridge to reimagine and declutter its QA programme, enhancing patient safety while baking in workflow efficiencies thanks to new-found opportunities for the automation of essential QA checks.
“Using a single QA vendor has helped us to streamline our staff training and ensure that specialist product knowledge is distributed consistently across the radiation therapy department,” adds Martin. That shared understanding is particularly important when running a medical physics service – like Clatterbridge does – across multiple sites and multiple teams. “What’s more,” notes Martin, “the synchronized approach to QA means patients will be treated using the same QA protocols regardless of location or the staff performing the QA. It also allows us to optimize our resource allocation collectively, with medical physics staff able to transfer more easily between Clatterbridge sites to provide cover as needed – a big plus throughout the COVID-19 pandemic.”
The QA engine-room
If that’s the back-story, what of the specifics? A key driver for Clatterbridge’s tie-up with Sun Nuclear is targeted improvement and innovation across the patient QA workflow, with automated first-fraction validation for every patient and automated in vivo monitoring of minor anatomical changes between fractions. On the machine side of the workflow, meanwhile, the ongoing priority is to remove subjectivity and manual analysis from image-based machine QA, driving efficiencies and standardization throughout the Clatterbridge radiotherapy network. Automating the machine QA workflow also yields significant time savings and simplification for daily, monthly and annual QA checks and compliance.

The SunCHECK Quality Management Platform provides what Clatterbridge needs through two core modules. SunCHECK Patient encompasses all aspects of patient QA, including secondary checks, phantomless pretreatment QA and automated in vivo monitoring. Meanwhile, SunCHECK Machine addresses critical machine QA needs, including template-driven daily, monthly and annual QA; automated imaging, multileaf collimator and volumetric modulated arc therapy (VMAT) checks; as well as long-term data trending and analysis.
Following implementation of the EPID-based SunCHECK Patient, the Clatterbridge medical physics team is now able to validate that the first fraction prescribed for its highest-risk patients matches the predicted transit dose, while automation of data processing in the background alleviates the need for user input. That’s especially important for patients receiving stereotactic body radiotherapy (SBRT), where there are only three to five higher-dose fractions and a higher degree of machine modulation versus standard treatment modalities – all of which means less room for error and, in turn, an additional layer of patient QA to maximize patient safety.
Another goal with SunCHECK Patient is to streamline workflows for the tracking of anatomical changes, especially patient weight loss, during a course of treatment. As part of a research study to road-test the software, Clatterbridge can now set its baseline as the first fraction or use SunCHECK Patient’s “predicted image” feature, tracking subsequent fractions against that baseline automatically. After some sensitivity testing, the Clatterbridge team has also defined a potential threshold that matches the current manually intensive help-desk triage system. Full clinical roll-out of the automated functionality is already under way, so that over time the team will review weight-loss issues only when a flag arises in SunCHECK Patient, enabling a more objective approach to identify critical cases for replanning.
“When it comes to patient QA, we simply couldn’t do manually what SunCHECK Patient does automatically,” notes Martin. The same goes for SunCHECK Machine and the automation of daily, monthly and annual QA tasks (versus the high levels of manual input required previously). “We have dramatically expanded the amount of patient and machine QA that we do,” he adds, “though it’s taking us much less time to do it because of the high degree of automation as well as the in-built accessibility that comes from a web-based software platform.”
It’s all about the numbers
What makes SunCHECK unique then is the ability to automate QA processes where the level of automation was previously non-existent. In a presentation at the recent ESTRO 2021 Annual Congress in Madrid, Spain, Martin and his colleagues quantified the efficiencies realized at the sharp-end of treatment delivery following the clinical roll-out of SunCHECK Machine at Clatterbridge (see table). Their study evaluated the automated QA software solution across nine linacs over a 12-month period, noting that automation of hitherto manually intensive QA measurements yielded significant aggregate time savings – 22 hours and 43 mins per linac per year – while an improvement in the analysis of visual tasks was immediately apparent as well.
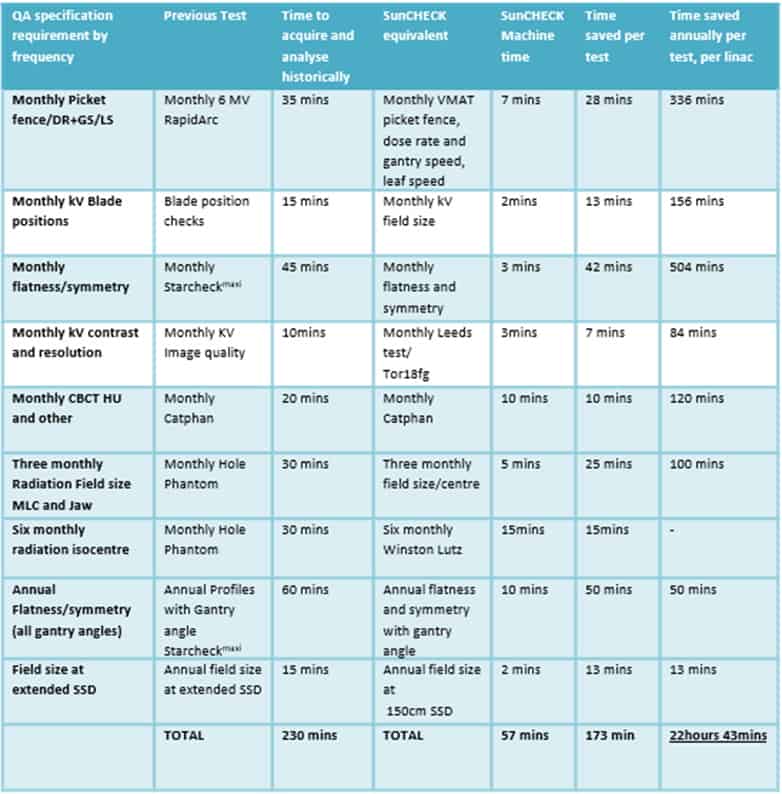
“A lot of the analysis we used to do on things like CatPHAN phantoms, picket fences and VMAT QA was done manually – drawing on regions of interest and entering in results,” notes Martin. “Overhauling and automating that process with SunCHECK Machine resulted in huge time-savings and quality benefits from the start…while the simplification of the workflow means that technicians can now manage the machine QA rather than physicists.”
Looking longer term, Clatterbridge’s drive for continuous improvement across its QA workflows will track developments on the SunCHECK platform, with a sustained pipeline of new features being rolled out across three or four new software releases each year. “It really helps that Sun Nuclear is open and collaborative when it comes to the product roadmap,” concludes Martin. “As such, the team here is, and will continue to be, an active part of the SunCHECK requirements-gathering conversation.”




