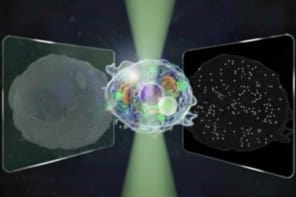
Researchers at the Massachusetts Institute of Technology (MIT) have developed a new dark-field imaging technique that does not require specialized microscope components. The technique, dubbed “substrate luminescence-enabled dark-field imaging” (SLED), involves adding a mirrored substrate to the sample stage of a standard optical microscope, and its simplicity could make dark-field imaging more widely accessible.
Dark-field microscopy produces high-contrast images of samples such as blood cells, bacteria, algae and marine organisms that are often transparent and provide little to no light absorption contrast. In a typical method, light is shone onto the microscope’s sample stage at a steep angle with respect to the sample surface normal. Because these highly oblique angles are larger than the maximum light collection angle of the microscope’s objective lens, the microscope only collects light that is scattered by the sample onto a cone centred around the instrument’s optical axis. This scattered light creates an image of the sample’s features that are in bright contrast to the dark background.
To create such images, however, researchers need to fit standard optical microscopes with specialized filter cubes. They must also use dedicated objectives or condensers to shape the incident light cone, adding to the expense and bulk of the instrument.
A luminescent photonic surface
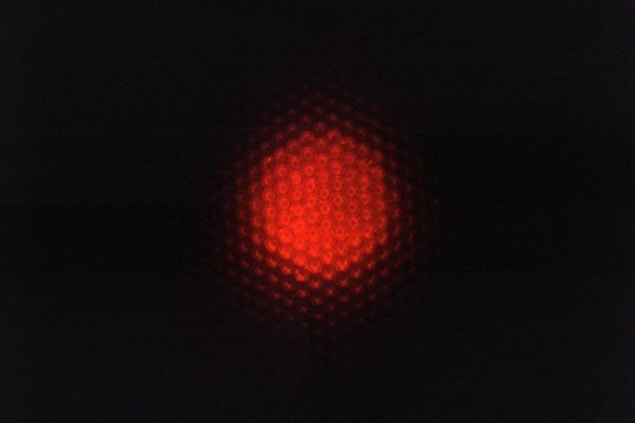
A team led by Matthias Kolle and Cecile Chazot of the Mechanical Engineering Department at MIT has now succeeded in integrating the components needed to create the cone of light for dark-field illumination into the surface on which the sample is placed. In the MIT instrument, this surface – the sample substrate – is made from a luminescent photonic material that emits light only at high angles as measured from the substrate surface’s normal. This light, Kolle explains, can only enter the microscope objective if it is scattered by the sample. Regions where there is nothing to scatter light (for instance, just water) will appear dark, while – as in conventional dark-field imaging – the features of small aquatic organisms, bacteria and other hard-to-image micron-sized objects appear bright. The substrate is thus “self-contained” and allows for dark-field contrast without the need for additional components.
How it works
The light emitted by the substrate is confined to high polar angle ranges thanks to the interplay between three different modules, Kolle says. The first module is a light source with a narrow spectral range (in this case, red). In the MIT experiments, this source consisted of core-shell semiconducting quantum dots (made from cadmium selenide/cadmium sulphide dispersed in a polymer matrix), but the researchers say that simple light-emitting diodes (LEDs) could work equally well.
This light source is positioned beneath a second module, comprising a spectrally selective mirror that allows only light of specific wavelengths to pass through, and only in specific directions. This mirror is made from alternating nanoscale layers of transparent materials with different refractive indices, which means they reflect incoming light at different angles.
“We tune this mirror so that it allows the red light generated by the quantum dots to pass through only at high angles (with respect to the surface normal),” explains Kolle. “If the light hits the mirror at the wrong angles, it bounces back and doesn’t escape from the substrate.”
Bragg reflector “gatekeeper”
In effect, this mirror – known as a Bragg reflector after the father-and-son team who established the underlying theory of how it functions – acts like a “gatekeeper”, Kolle says. It only permits light of a given colour to escape from the substrate at specific angles.
But what happens to the light that isn’t allowed to escape or light that is emitted by the quantum dots far from the Bragg reflector? For that, the researchers added a third module below the light source – a micropatterned mirror containing small wells around 4 microns across. This mirror bounces light back towards the top Bragg reflector and redirects it so that it has the chance to hit the reflector at an angle at which it can escape. The mirror is moulded from solid transparent epoxy coated with a reflective gold film. According to Kolle, its design was inspired by the wings of the Papilio butterfly, which get their iridescent colour from their micron-scale structure.
Sending light onto the sample at the right angles
The researchers, who report their work in Nature Photonics, say they have already used their technique to image individual bacterial cells and microorganisms in seawater. They add that the crucial photonic substrate could be mass-produced with existing techniques, and that it could be integrated into even the most simple and compact optical microscopes. It might also be incorporated into miniature dark-field imaging devices for applications in point-of-care medical diagnostics and bio-analytics. Kolle says his team is working on prototypes for such applications.