The use of tissues with cells previously removed (decellularized) is gaining traction in tissue engineering research. These decellularized grafts serve as a scaffold for new cells to grow in an environment similar to native conditions, because decellularization minimally affects the tissue microstructure. The preservation of this microstructure and other functional compounds is believed to enhance cell attachment and proliferation and, hence, healing of the tissue.
Now, researchers from the Department of Plastic Surgery at Shanghai Jiao Tong University, led by Guangdong Zhou, have shown that these constructs promote faster and better healing in cartilage defects in a porcine model (Biomed. Mater. 13 025016). Furthermore, when adding cells from the host into the decellularized construct before implantation, they observed superior results.

Is animal tissue a suitable material?
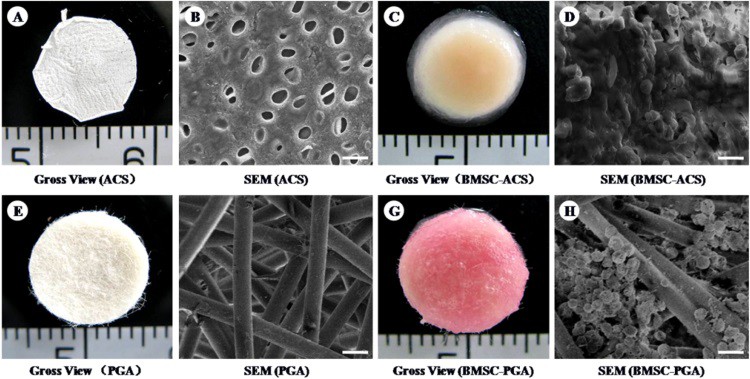
Although the use of decellularized tissues for cartilage repair has been reported in numerous small-animal studies, fewer groups have studied their implantation in large animals. In this study, the researchers produced decellularized cartilage from pigs, characterized it and implanted it in cartilage defects of different pigs – a process known as allotransplantation (transplantation between individuals of the same species). They used poly-glycol acid (PGA), a widely used polymer in research and clinics, as a control.
After decellularization, the processed tissues maintained their structure and some biofunctional compounds, such as growth factors – molecules able to promote cell metabolism and proliferation. This finding was crucial to prove the maintenance of the microstructure and biofunctionality of the processed tissue. In addition, the processed tissues presented a higher compatibility with cells than the polymer scaffolds.
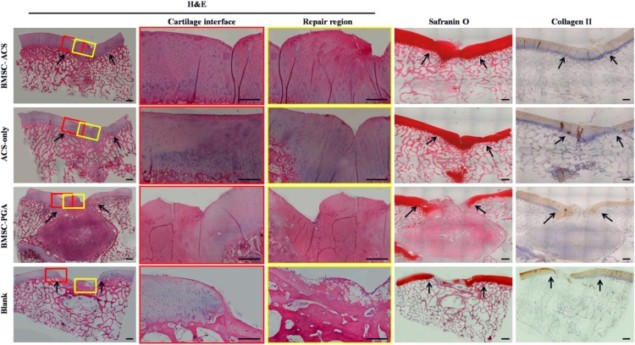
Decellularized cartilage performs the best
Next, the researchers implanted three materials into created cartilage defects: decellularized cartilage loaded or unloaded with stem cells derived from the bone marrow of the same animal, or PGA scaffolds loaded with stem cells. This enabled them to analyse three different conditions, together with a non-treated group used as a control. Interestingly, unloaded decellularized tissue provided better healing than the cell-loaded PGA material, exhibited by better tissue regeneration and recovery of mechanical compressive properties. This suggests that unloaded decellularized scaffolds trigger a better host response than polymer scaffolds, even ones loaded with the animal’s own cells.
However, the most promising results originated from the implanted decellularized cartilage loaded with stem cells. After implantation, this material was able to promote the shift of stem cells into cartilage cells, a process named “chondrogenic differentiation”, which the authors related to a superior healing than the rest of the conditions. This confirmed the potential of allografts in combination with autologous cells (cells coming from the patient) for treatment of degenerated cartilage.
Future perspectives: a long road to walk
Despite the positive results observed in this study, the authors point out that “the underlying mechanisms … are still unknown”. In addition, the use of decellularized tissues is a double-edged-sword, and many issues like “biosafety and long-term fate” still need to be investigated. Nonetheless, the current study makes an important step in the path towards achieving complete regeneration after the treatment of cartilage defects, although there is still a long way to go.



