Fluorescence imaging is a valuable method for examining biological systems. To achieve the maximum tissue penetration depth and minimum light scattering, detecting near-infrared (NIR) fluorescence in the long-wavelength end of the second NIR window (1500–1700 nm), known as NIR-IIb, provides the best results. Unfortunately, NIR-IIb imaging relies on nanoparticle fluorescent probes that often contain toxic elements, hindering its clinical translation.
Biocompatible small-molecule NIR fluorescent probes do exist. Indocyanine green (ICG), for example, is approved by the US Food and Drug Administration and has already been used for clinical applications. Such small-molecule fluorophores, however, emit in the shorter-wavelength NIR-I and NIR-IIa windows (700–1000 and 1000–1300 nm). And light scattering at these wavelengths limits the imaging depth and causes low contrast images.
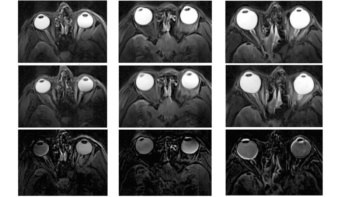
To achieve high image contrast and clarity while using biocompatible probes, Zhuoran Ma, his PhD adviser Hongjie Dai, and colleagues at Stanford University turned to deep learning. Using roughly 2800 in vivo images of mice taken in the NIR-IIa and NIR-IIb windows, they trained artificial neural networks to transform blurred NIR-IIa fluorescence images into higher-resolution images previously only achievable using NIR-IIb.
“The main application for NIR imaging will be diagnosis of cancer and image-guided tumour surgery,” explains Ma. “Compared to other imaging modalities such as CT or MRI, NIR imaging allows real-time imaging, which can help pinpoint the tumour site during surgery.”
In vivo investigations
To assess their image-processing method, the researchers injected a mouse with a NIR-IIa probe and an NIR-IIb fluorophore, and recorded fluorescence images of the animal’s blood vessels in the NIR-IIa and NIR-IIb windows. Processing the NIR-IIa image with the trained neural network generated an image that resembled the ground-truth NIR-IIb image, demonstrating that the generator could reliably enhance image contrast without introducing artefacts.
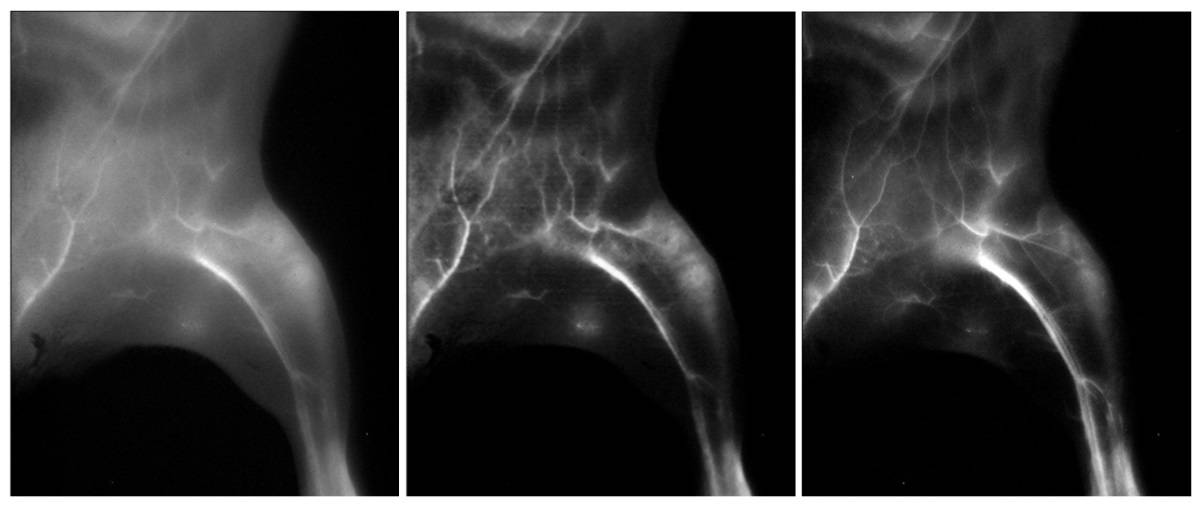
Next, the team used the trained neural network to transform wide-field NIR-IIa fluorescence images of lymph nodes in a mouse injected with the FDA-approved small-molecule dye ICG and an NIR-IIb nanoparticle probe. The neural network increased the signal-to-background ratio of superficial sacral lymph node images from 8.44 to 117.0, a level that could enable sentinel lymph node mapping in the clinic. The researchers note that the generated high-resolution images closely resembled the NIR-IIb images.
Interestingly, the signal-to-background ratio of lymph node images in the NIR-I window, a modality that’s currently being used in clinical trials, was also improved by the neural network, even though NIR-I images were not used for training.
Ma and colleagues also demonstrated that the neural network significantly enhanced molecular imaging of a mouse tumour model using a tumour-targeting fluorophore–antibody complex. The transformed images had tumour-to-normal tissue ratios of 18.2 in the NIR-I window and 25.3 in the NIR-IIa window, up to five times higher than in the original images. Such results could one day enable fluorescence imaging for cancer diagnosis or guided resection of tumours.
Finally, the researchers showed that their translation algorithm could enhance NIR-II light-sheet microscopy (LSM), a recent development that provides non-invasive in vivo volumetric optical imaging of mouse tissues. They demonstrated that generated LSM images exhibited similar signal-to-background ratio and vasculatures sizes to ground-truth NIR-IIb LSM images, increasing the depth limit of one-photon LSM in the NIR-IIa window from below 2 mm to around 2.5 mm.
Non-invasive NIR imaging tracks brain shrinkage
The researchers conclude that this ability to generate high-resolution images from scattering-blurred NIR images could open new opportunities for clinical translation. “Instead of trying to improve the biocompatibility and alleviating the toxicity of the nanoparticle-based NIR-IIb fluorophores, one could apply FDA-approved molecules directly and transform the low-resolution images to high-resolution ones, which would be an excellent application of artificial intelligence,” they write.
The team is now aiming to extend this method for use in larger animals such as rats. “Ultimately, we hope this can be applied in real clinics for human use,” Ma tells Physics World.
The research is described in Proceedings of the National Academy of Science.