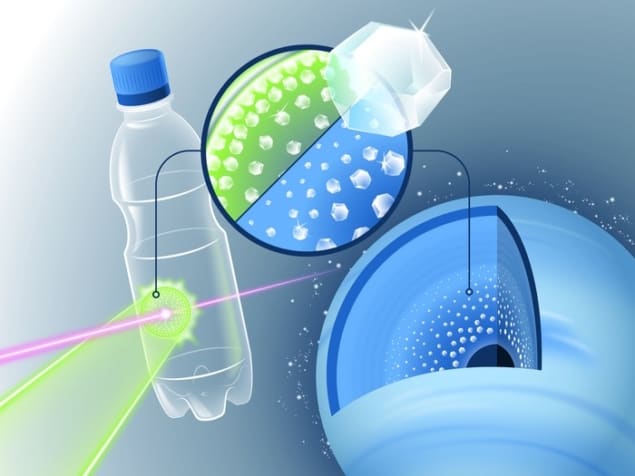
Firing powerful laser pulses at pieces of plastic has provided new insights into how diamonds could form and rain down on ice-giant planets such as Neptune and Uranus. The experiment by researchers in Germany, France and the US could also lead to a better industrial process for making diamonds here on Earth.
Team member Dominik Kraus at the University of Rostock explains that the group used energetic pulsed optical lasers to drive a shock compression wave into a film of PET plastic. The pressure of the wave was about one million times Earth’s atmospheric pressure, which simulates conditions a few thousand kilometres beneath the surface of ice giants like Neptune and Uranus. The shock wave only travels for a few nanoseconds, but that was enough time for the team to use femtosecond pulses from X-ray free electron lasers to make “movies” of the chemical processes inside the shock-compressed samples.
“We used two main diagnostic techniques,” says Kraus. “X-ray diffraction, which showed us that diamond crystal structures are forming, and small angle X-ray scattering, which provided the in-situ size distribution of the diamonds created.” He adds that the combination of these two techniques in a single experiment is an extremely powerful way of characterizing chemical reactions under such extreme conditions.
Ice giants and plastic bottles
PET is the same material used in plastic bottles, but in this case a simple PET film was used rather than the thicker material found in bottles.
“We used PET plastics because it includes a mixture of light elements which are thought to be the main constituents of the icy giant planets: hydrogen, carbon, oxygen,” says Kraus. “At the same time, PET is stoichiometrically a mixture of carbon and water. We wanted to tackle the question of whether diamond precipitation can happen via demixing of carbon and hydrogen in the presence of oxygen.”
As well as providing important insights into chemical processes that occur on these distant planets, the research also provides clues about how ice giants can form magnetic fields. Earth’s magnetic field is created by the motion of liquid iron in our planet’s outer core. Uranus and Neptune have very different magnetic fields, which some planetary scientists believe are generated much closer to the planets’ surfaces by superionic water. In this form of water, the oxygen atoms form a crystal lattice through which hydrogen ions can flow like a fluid and therefore generate magnetic fields.
“We have not seen direct evidence for the formation of superionic water in these experiments as the pressure was probably too low,” says Kraus. “However, the observed demixing of carbon and water certainly points to the formation of superionic water in planets like Uranus and Neptune.”
Industrial diamonds
The research could also have important implications for the industrial production of diamonds.
“In our experiment the diamonds reached sizes of about 2–5 nm,” says Kraus. “This is just a few 100 to a few 1000 carbon atoms. That’s more than 10,000 times smaller than the thickness of a human hair. It should be noted that in our experiments the diamonds only have nanoseconds to grow. This is why they are so small. In planets, they will of course grow much larger within millions of years.”
Superionic ice phases could explain unusual magnetic fields around Uranus and Neptune
As it stands, the method used in this experiment does not produce enough nanodiamonds to come close to being a practical industrial process. However, Kraus points out that the new technique is much cleaner than the current method of using explosives to produce industrial nanodiamonds. These explosive processes are difficult to control and dirty in comparison to the laser shock compression of plastics. While it is unlikely that we will be digging bottles out of the landfill to turn them into diamonds on an industrial scale, Kraus believes this process could become much more efficient than current methods.
“Currently, we create only a few micrograms of nanodiamonds per laser shot,” says Kraus. “But the revolutionary increase in shot rates of those lasers should allow the production of macroscopic quantities.”
The research is described in Science Advances.