Preterm births account for about 10% of all births in the US and according to the World Health Organization, this number is increasing rapidly. The survival rate for babies with a gestational age of 28 weeks or less is lower than 50% with respiratory disease syndrome being the second major cause of death. This is because lungs are among the last organs to fully develop. One of the main challenges here is to deliver oxygen to the new-borns using external devices, such as mechanical pumps, until their lungs are fully formed, but these ventilators can cause serious problems in themselves.
Researchers at McMaster University in Canada led by P. Ravi Selvaganapathy and Paracelsus Medical University in Germany led by Christoph Fusch have now developed a passive lung device that is pumped by the baby’s own heart (in which the arterio-venous pressure differential is between just 20 and 60 mmHg). Such a device is known as an “artificial placenta” and consists of microchannels that efficiently exchange oxygen between the blood and outside air. Such a device would be connected to the umbilical cord of the new-born.
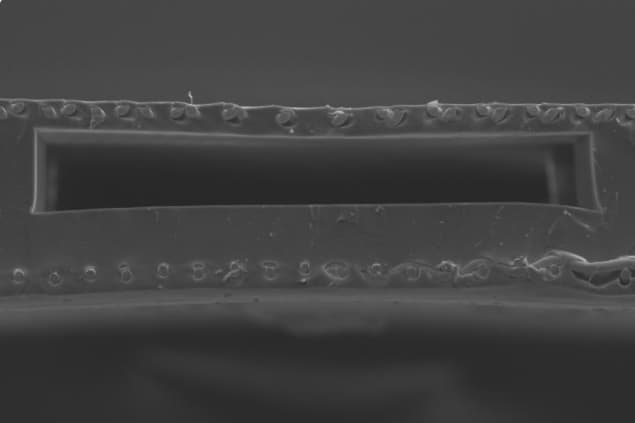
Although the concept itself is not new, the design developed by Selvaganapathy and colleagues makes use of both sides of the microchannel network for gas exchange (as opposed to just one side as in previous devices). This significantly increases the surface area to volume ratio of the device.
343% better in terms of oxygen transfer
The highest-performing “double-sided single oxygenator units” (dsSOUs) that the researchers made were about 343% better in terms of oxygen transfer compared to single-sided SOUs with the same height. They used their design to make a prototype containing a gas exchange membrane with a stainless-steel reinforced thin (50-micron-thick) PDMS layer on the microchannel network. This design was based on a previous one developed in their lab (Biomicrofluidics 12 014107).
In the present work, they succeeded in incorporating a slightly thicker (150-micron) steel reinforced membrane on the other side of the blood channels to increase gas exchange. The new fabrication process means a 100% increase in the surface area for gas exchange while continuing to mimic the placenta by ensuring that the priming volume (how much blood is removed at a time for oxygenation) remains low.
“The key innovation here is developing a large-area microfluidic device,” says Selvaganapathy. “You want it to be microfluidic because a 1-kg baby, for example, might only have 100 ml of blood. You want a device to use only a one-tenth of that volume at a time.”
Meeting 30% of the oxygenation needs of a preterm neonate
The team has already produced an optimized oxygenator to build a lung assist device (LAD) that could meet 30% of the oxygenation needs of a preterm neonate weighing between 1 and 2 kg. The LAD provides an oxygen uptake of 0.78-2.86 ml/min, which correspond to an increase in oxygen saturation from around 57 to 100% in a pure oxygen environment.
Crystal phase-transitions keep babies in third-world countries warm
Reporting on their work in Biomicrofluidics 12 044101, the researchers say that the design could be improved by coating the surface of the dsSOUs with antithrombin-heparin or polyethylene glycol to improve the anticoagulation properties of PDMS surfaces, which are in contact with blood. “Finally, new designs that have higher gas exchange can be used to provide the sufficient oxygenation in ambient air,” they write.
Jeffrey Borenstein of the Charles Stark Draper Laboratory in Cambridge, Massachusetts, who was not involved in this work says that this new study “targets an extremely exciting and promising opportunity in artificial organs research, using microfluidics technology to overcome many of the current limitations of respiratory assist devices.
“Selvaganapathy and colleagues’ advance points to microfluidics as a means to reduce the incidence of clotting, miniaturize the device, and potentially operate the system on room air to enable portable and wearable systems,” he tells Physics World.




