Full-thickness skin wounds, especially those caused by burns, require skin grafts to fully heal and prevent infections. An ideal skin substitute for such grafts should have similar mechanical properties to human skin, support cell attachment and proliferation, degrade at a comparable rate to the formation of new skin, and prevent infections. To date, no such product exists.
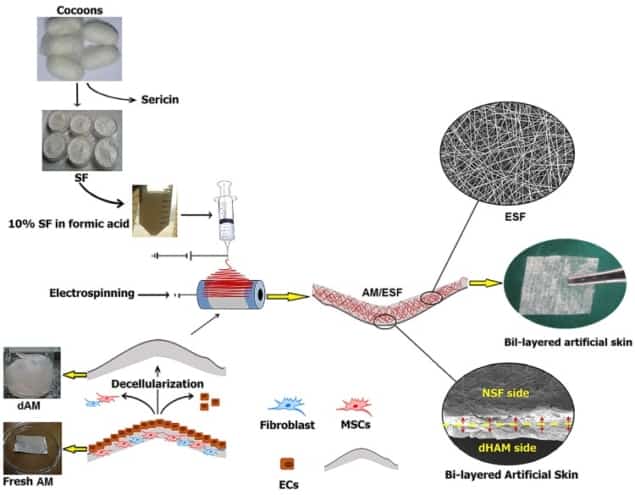
To address this shortfall, a research collaboration from Iran, UK, USA and Portugal is developing a novel skin substitute based on decellularized human amniotic membrane (AM). AM is a natural bio-scaffold that offers high elasticity and structural integrity, antibacterial activity and support for cell growth. While AM is widely used to manage burn wounds, its weak mechanical properties and rapid degradation make it less than optimal. By coating AM with silk protein, the researchers hope to overcome these disadvantages (Biomed. Mater. 13 035003).
“Burn injury – from fire, battlefield or acid attack – has been reported as an important cause of morbidity and mortality and is still considered an unmet clinical need,” explained Alexander Seifalian, from the Nanotechnology and Regenerative Medicine Commercialisation Centre, The London BioScience Innovation Centre.
Stable structure
Seifalian and co-workers – including first author Mazaher Gholipourmalekabadi – fabricated the artificial skin by electrospinning nanofibrous silk fibroin solution onto decellularized AM. After electrospinning for 20 minutes, scanning electron microscopy (SEM) revealed that the electrospun silk fibroin (ESF) nanofibers had successfully collected on the AM. For further experiments, the team created AM/ESF bilayer membrane samples by electrospinning for 3 hr, followed by ethanol treatment for 1 hour to induce transition into an insoluble β-sheet conformation.
The researchers first evaluated the biomechanical behaviour of AM and AM/ESF samples. They found that the AM/ESF bilayer showed significantly improved mechanical and viscoelastic properties – including maximum load value, suture retention strength, strain deflection at break and thickness – compared with AM samples.
The degradation rate of tissue scaffolds can profoundly affect healing effectiveness. If a biomaterial degrades too quickly the scaffold may disintegrate before the damaged tissue is healed. The team tested the in vitro degradation rates of AM and AM/ESF, and observed that coating the AM with ESF slowed the degradation rate of the resulting bilayer membrane. “ESF keeps the biological structure in place for regeneration of skin while the AM gradually bioabsorbs,” Seifalian explained.
Stem cell seeding
One of the most important characteristics of scaffolds used in tissue engineering is their ability to support cell attachment and growth. As such, the researchers examined the growth of adipose tissue-derived mesenchymal stem cells (AT-MSCs) on AM samples and on AM/ESF before and after ethanol treatment.
The researchers first used SEM to analyse the morphology of AT-MSCs cultured on the various substrates. Three days post-seeding, they clearly observed the spindle morphology of the AT-MSCs on all samples, demonstrating effective cell-substrate attachment. All samples exhibited similar cell density, implying that both AM and AM/ESF offer desirable cell adhesion properties for tissue engineering applications.
They also examined the long-term cell viability and cytotoxicity, using MTT and LDH assays, respectively. They found that neither AM nor AM/ESF affected the viability of the AT-MSCs after specific incubation intervals, and that neither sample conferred any cytotoxic effects on the cells.
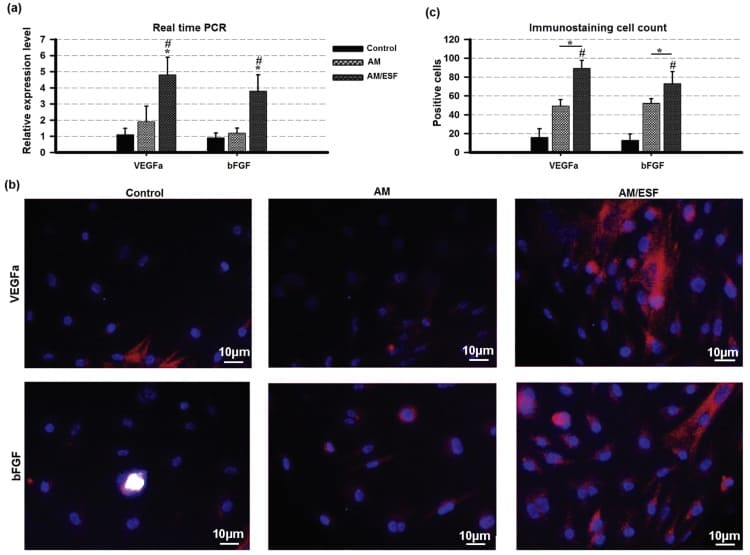
Finally, the team investigated whether AM/ESF could accelerate blood vessel growth after injury, following previous reports that AM possesses anti-angiogenic properties. They seeded AT-MSCs on AM and AM/ESF and incubated the samples in 5% CO2 and 95% air for seven days. They observed that AM/ESF significantly increased expression of the pro-angiogenic VEGFa and bFGF from the AT-MSCs, compared with the AM, indicating that the ESF coating enhanced angiogenesis in vitro.
“We have shown that the AM/ESF bilayer membrane provides a good microenvironment for growth and attachment of AT-MSCs,” said Seifalian. “We suggest this membrane as a promising cell delivery system for scaffold/cell-based therapy.”
Next, the team plan to evaluate the AM/ESF artificial skin in vivo in a rodent model, then if the outcome is satisfactory, they will start GLP (good laboratory practice) preclinical evaluation. “At this stage, we will also talk to the regulatory body, MHRA in the UK, with regard to their requirements for a clinical feasibility study,” Seifalian told medicalphysicsweb.