A patient’s disease state can be revealed via detection of biomarkers (molecules that are measurable indicators of specific diseases) in their bloodstream. Biomarker detection normally requires a blood sample to be drawn from the patient and sent to a specialized laboratory. A primary goal of cutting-edge biomarker-detection research is to bring the lab to the patient instead, using devices that can continuously monitor a patient’s blood and provide real-time information on the presence and concentration of biomarkers.
As such, many researchers are investigating new strategies for continuous biomarker detection based on simple, durable and compact sensors with high sensitivity. For a team of researchers led by Menno Prins at Eindhoven University of Technology, the solution to this problem is something you may carry around in your pocket: the camera.
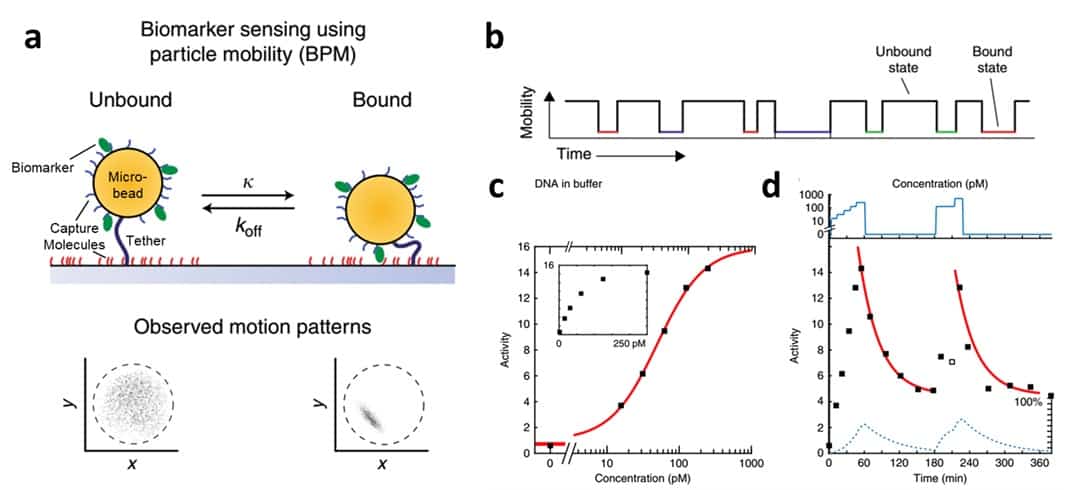
The challenge in using conventional cameras to detect biomarkers is figuring out how to create a detectable signal from molecules that are too small to photograph. In their recent paper, the team report a clever trick to detect biomarkers by monitoring the mobility of specially engineered microbeads that are large enough to photograph using a simple magnifying lens (Nature Communications 9 2541). They call their technique “biomarker sensing using particle mobility” (BPM).
In BPM, each microbead is attached to a glass surface via a long molecular tether and, under normal conditions, floats around in an area that is defined by the length of the tether. The microbead is coated with a “capture molecule” that binds to a biomarker of interest, and the glass surface is coated with another capture molecule that binds to a different side of the same biomarker. As a single biomarker molecule sticks to both the microbead and the surface simultaneously the particle is pinned down and its motion is significantly restricted.
After exposing the microbeads to a patient’s blood, a conventional camera can be used to simultaneously monitor hundreds of microbeads as they transition from mobile to immobile. A short five-minute video can then be processed to translate information about the average particle mobility to information about the amount of biomarker present in the blood sample. Specifically, the team defined “activity”, which is related to the frequency at which particles transition between high-mobility and low-mobility state, as a metric to quantify biomarker concentration.
The researchers demonstrated that BPM could detect dilute concentrations (nanomolar to picomolar) of DNA- and protein-based biomarkers in conventional buffers and even in human blood plasma, establishing BPM as a sensitive and robust technique. As they flowed a DNA biomarker with concentrations in the picomolar range over the sensor surface, the measured activity increased.
A fundamental requirement for any continuous bloodstream biomarker sensing system is reversibility – the sensor needs to recover to baseline signal once the biomarker concentration is reduced to zero. To test the reversibility of their technique, the team added a biomarker, flushed it out, and then added the biomarker again. They indeed found that particle activity returned to near-baseline after biomarker removal and then increased again upon re-addition of biomarker.
Moving forward, the researchers hope to expand BPM’s capabilities to include the detection of more diverse biomarkers (proteins and small molecules, for example), and to implement BPM in a miniaturized, portable device.



