
Surgical sutures are strong, flexible fibres used to close wounds caused by trauma or surgery. But could these stitches do more than just hold wounds closed? Could they, for example, be designed to accelerate the healing process?
A research team headed up at Donghua University in Shanghai has now developed sutures that can generate electricity at the wound site. They demonstrated that the electrical stimulation produced by these sutures can speed the healing of muscle wounds in rats and reduce the risk of infection.
“Our research group has been working on fibre electronics for almost 10 years, and has developed a series of new fibre materials with electrical powering, sensing and interaction functions,” says co-project leader Chengyi Hou. “But this is our first attempt to apply fibre electronics in the biomedical field, as we believe the electricity produced by these fibres might have an effect on living organisms and influence their bioelectricity.”
The idea is that the suture will generate electricity via a triboelectric mechanism, in which movement caused by muscles contracting and relaxing generates an electric field at the wound site. The resulting electrical stimulation should accelerate wound repair by encouraging cell proliferation and migration to the affected area. It’s also essential that the suture material is biocompatible and biodegradable, eliminating the need for surgical stitch removal.
To meet these requirements, Hou and colleagues created a bioabsorbable electrical stimulation suture (BioES-suture). The BioES-suture is made from a resorbable magnesium (Mg) filament electrode, wrapped with a layer of bioabsorbable PLGA (poly(lactic-co-glycolic acid)) nanofibres, and coated with a sheath made of the biodegradable thermoplastic polycaprolactone (PCL).
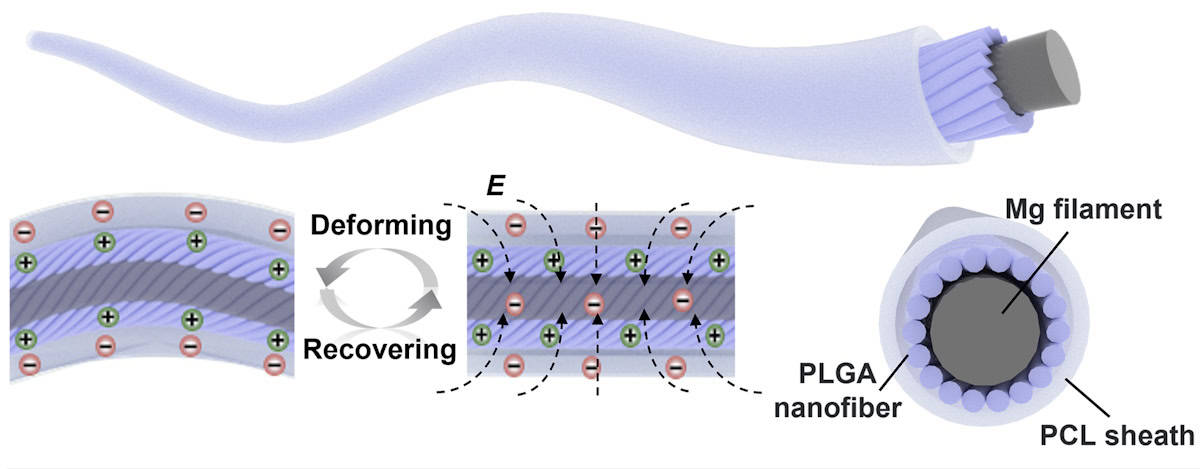
After the BioES-suture is used to stitch a wound, any subsequent tissue movement results in repeated contact and separation between the PLGA and PCL layers. This generates an electric field at the wound site, the Mg electrode then harvests this electrical energy to provide stimulation and enhance wound healing.
Clinical compatibility
The researchers measured the strength of the BioES-suture, finding that it had comparable sewing strength to commercial sutures. They also tested its biocompatibility by culturing fibroblasts (cells that play a crucial role in wound healing) on Mg filaments, PLGA-coated Mg and BioES-sutures. After a week, the viability of these cells was similar to that of control cells grown in standard petri dishes.
To examine the biodegradability, the researchers immersed the BioES-suture in saline. The core (Mg electrode and nanofibre assembly) completely degraded within 14 days (the muscle recovery period). The PCL layer remained intact for up to 24 weeks, after which, no obvious BioES-suture could be seen.
Next, the researchers investigated the suture’s ability to generate electricity. They wound the BioES-suture onto an artificial muscle fibre and stretched it underwater to simulate muscle deformation. The BioES-suture’s electrical output was 7.32 V in air and 8.71 V in water, enough to light up an LCD screen.
They also monitored the BioES-suture’s power generation capacity in vivo, by stitching it into the leg muscle of rats. During normal exercise, the output voltage was about 2.3 V, showing that the BioES-suture can effectively convert natural body movements into stable electrical impulses.
Healing ability
To assess the BioES-suture’s ability to promote wound healing, the researchers first examined an in vitro wound model. Wounds receiving electrical stimulation from the BioES-suture exhibited faster migration of fibroblasts than a non-stimulated control group, as well as increased cell proliferation and expression of growth factors. The original wound area of approximately 69% was reduced to 10.8% after 24 h exposure to the BioES-sutures, compared with 32.6% for traditional sutures.
The team also assessed the material’s antibacterial capabilities by immersing a standard suture, BioES-suture and electricity-producing BioES-suture in S. aureus and E. coli cultures for 24 h. The electricity-producing BioES-suture significantly inhibited bacterial growth compared with the other two, suggesting that this electrical stimulation could provide an antimicrobial effect during wound healing.
Finally, the researchers evaluated the therapeutic effect in vivo, by using BioES-sutures to treat bleeding muscle incisions in rats. Two other groups of rats were treated with standard surgical sutures and no stitches. Electromyographic (EMG) measurements showed that the BioES-suture significantly increased EMG signal intensity, confirming its ability to generate electricity from mechanical movements.
After 10 days, they examined extracted muscle tissue from the three groups of rats. Compared with the other groups, the BioES-suture improved tissue migration from the wound bed and accelerated wound regeneration, achieving near-complete (96.5%) wound healing. Tissue staining indicated significantly enhanced secretion of key growth factors in the BioES-suture group compared with the other groups.
The researchers suggest that electrical stimulation from the BioES-suture promotes wound healing via a two-fold mechanism: the stimulation enhances the secretion of growth factors at the wound; these growth factors then promote cell migration, proliferation and deposition of extracellular matrix to accelerate wound healing.
Smart stitches could prevent infections at surgical sites
In an infected rat wound, stitching with BioES-suture led to better healing and significantly lower bacterial count than wounds stitched with ordinary surgical sutures. The bacterial count remained low even without daily wound disinfection, indicating that the BioES-suture could potentially reduce post-operative infections.
The next step will be to test the potential of the BioES-suture in humans. The team has now started clinical trials, Hou tells Physics World.
The BioES-suture is described in Nature Communications.




