Available to watch now, The Electrochemical Society in partnership with Scribner and Hiden Analytical explore an environmentally friendly approach to upcycling CO2 emissions
Want to learn more on this subject?
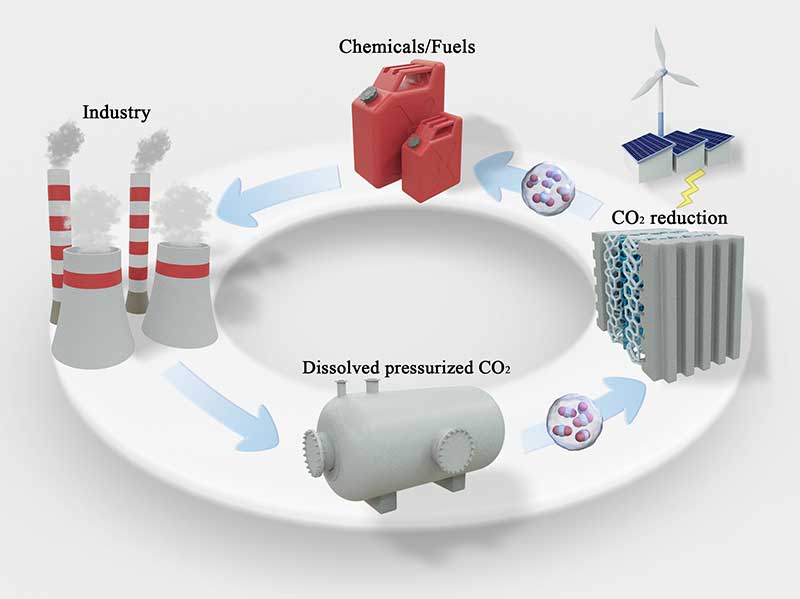
The electrochemical reduction of carbon dioxide (CO2R) is a strategic approach aimed at completing the carbon cycle for chemical production. Traditionally, this field has predominantly focused on conducting electrolysis on CO2 under standard atmospheric pressure. However, in industrial applications, CO2 is typically pressurized during its capture, transportation, and storage, often existing in a dissolved state.
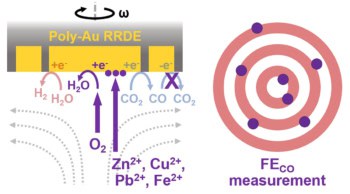
A significant discovery has been unveiled: subjecting aqueous CO2 to a pressure of 50 bar alters the CO2R pathways, favouring the formation of formate. This phenomenon is consistently observed across commonly used CO2R catalysts. Through the development of effective techniques for operating under high pressures, including a measurable Raman spectroscopy approach during the ongoing reaction, a connection has been established between the increased preference for formate and the heightened coverage of CO2 on the cathode surface. This collaboration between theoretical models and experimental data strongly supports this mechanism and has led to enhancing the cathode surface of a copper electrode with a proton-resistant layer. This innovation amplifies the selective impact caused by pressure. Furthermore, research has unveiled the potential to transform gas-phase high-pressure CO2 into ethylene (C2H4) through CO2R. Density functional theory calculations were conducted to identify a range of copper alloys that promote C-C dimerization under high pressure, which represents the rate-limiting step for C2H4 production. Theoretical predictions were validated through a combination of electrochemical measurements and operando observations, guiding the design of a copper-based catalyst for efficient and active conversion of CO2 to C2H4.
An interactive Q&A session follows the presentation.
Want to learn more on this subject?

Xu Lu is assistant professor of Mechanical Engineering at the King Abdullah University of Science and Technology (KAUST). He is affiliated with the Clean Combustion Research Center (CCRC) and KAUST Solar Center (KSC). Lu’s Low-carbon Energy Conversion and Storage (LECS) Lab focuses on electrochemical conversion of high-pressure CO2 conversion. So far, the LECS Lab has generated two US provisional patents and research articles in Nature Communications (two), Angewandte Chemie, Chemical Engineering Journal, Joule, and others. The LECS lab is also developing cutting-edge pilot-scale showcases with industrial partners such as ACWA Power and Aramco. He obtained his BS and PhD from the Department of Mechanical Engineering at the University of Hong Kong in 2012 and 2017, respectively. He trained as a postdoctoral fellow in the Department of Chemistry at Yale University. Lu joined KAUST in March 2021.