Enzymes are biomolecules that perform specialized functions, such as the assembly or degradation of small molecules. The small size of enzymes (tens of nanometres) previously limited their study to analysis of the collective properties of large numbers (thousands or more). However, recent advances in nanotechnology have enabled researchers to look at individual enzymes one-at-a-time on the nanosecond time scale.
The team, led by Steven Granick, noted that this behaviour could be explained by a fundamental property of diffusion theory, which states that diffusive substances concentrate in areas in which they move more slowly. As such, they hypothesized that interactions with substrate molecules somehow caused enzymes to diffuse through solutions at greater speeds.
The researchers then went further in their reasoning: they theorized that when an enzyme catalyses a chemical reaction (the cleavage of a covalent bond, for example), some of the energy released in that chemical reaction could be converted into kinetic energy – giving the enzyme some linear momentum. By contrast, enzymes that don’t have substrate bound to them would travel through solution according to the laws of Brownian diffusion, taking smaller steps in random directions. This effect, which they called enzyme-leaping, would create a link between substrate concentration and average enzyme velocity, thus explaining the phenomenon of antichemotaxis.
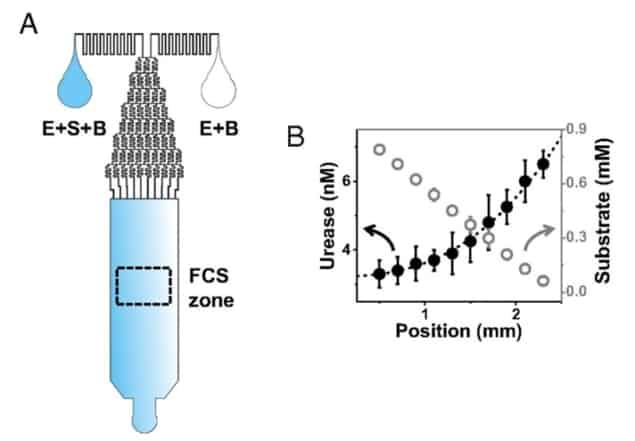
To validate this theory, Granick and colleagues investigated whether they could observe individual enzymes leaping through solution in the presence of substrate. To track individual enzymes, they used fluorescence correlation spectroscopy (FCS).
In FCS, each enzyme is linked to a small fluorescent molecule and a tiny laser beam (hundreds of nanometres wide) is used to visualize fluorescence in a very small volume of space. When an individual enzyme wanders into the laser beam, its linked fluorescent molecule emits light, which is captured using a photodetector. The fluorophore doesn’t stop fluorescing until the enzyme exits the space illuminated by the laser beam. As such, the enzyme transit time is defined by the length of time that the photodetector records brightness.
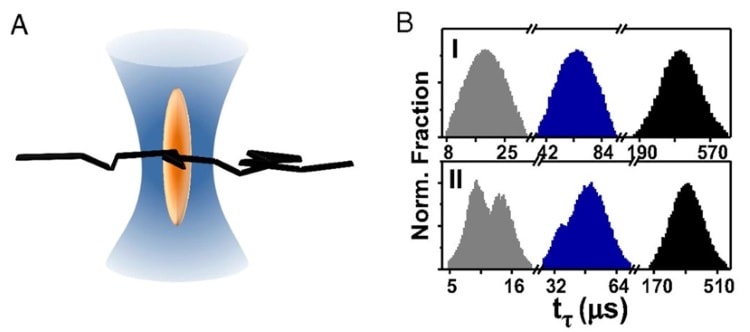
Using a state-of-the-art super-resolution technique, the team tuned the width of the laser beam down to 70 nm. Using this minimum beam width, the researchers observed two populations of transit times – one fast and one slow. They attributed the slow population to simple Brownian diffusion – just enzymes moving randomly through solution looking for substrate. However, the fast population exhibited statistical properties that were more consistent with ballistic motion.
This observation suggested that the fast population actually consisted of enzymes that were “leaping” linearly through the space illuminated by the laser beam. The team also found that decreasing substrate concentration decreased the size of the fast population, providing further support for their hypothesis by showing that enzyme leaps were linked to the presence of substrate.
When the researchers increased the width of the laser beam to above 100 nm, the fast population disappeared. This finding indicates that enzyme leaps have a length scale on the order of 50-100 nm, above which the enzyme’s momentum gets dissipated by viscous drag forces. Performing simple calculations, the team proposed that each enzyme kick is initiated by a spontaneous force roughly 1 piconewton in magnitude, transferring kinetic energy 10-20 times greater than thermal energy to the enzyme.
The exact cause of enzyme kicks is not understood. However, antichemotaxis, the property that appears to emerge directly from the existence of enzyme kicks, could have an important regulatory role in the spatial distribution of cellular biomolecules. “This can be biologically useful because it homogenizes the spatial distribution of the enzymatic production, which is essential in the crowded milieu of the cell,” the authors conclude.