Plants need iron (II) ions (Fe2+) to perform photosynthesis and produce chlorophyll. In nature, however, Fe2+ is much less common than its trivalent cousin Fe3+. A team of researchers from the East China University of Science and Technology in Shanghai has now found that iron in the fallen leaves of dead trees helps make up this shortfall by transforming Fe3+ to Fe2+ via charge transfer reactions. The result, obtained via a range of spectroscopic techniques, provides insights into the mechanisms behind this valence state change, and indicates that leaves decomposed in soil are an important source of much-needed Fe2+.
While plants naturally transform Fe3+ to Fe2+ via mechanisms such as reduction reactions that involve molecules produced in their roots, they can still suffer from Fe2+ deficiency. Shanshan Liang, a member of the study team, notes that this deficiency has serious consequences for agriculture. “The lack of Fe2+ in plants can leads to fewer photosynthetic components, interveinal chlorosis of young leaves and poor root formation, which all result in reduced crop yields,” she explains.
While inorganic fertilizers like FeSO4 are routinely used to increase the quantities of Fe2+ in soil, this is relatively inefficient because the Fe2+ quickly transforms into Fe3+ oxides. Mineral fertilizers, such as iron chelates, are better in this respect, but they are expensive. Genetically modifying crops so that they take up more Fe2+ from soil is also possible, but it introduces scientific and policy-related complications.
The cation-π interaction
In previous work, a team led by Haiping Fang discovered that iron changes its valence state during biochemical reactions due to charge transfer that occurs during a process called chelation. This process takes place between Fe3+and certain enzymes produced in the roots of plants, and possible charge transfer mechanisms include a direct transfer of charge from donor atoms to cations. This direct transfer is known as the cation-π interaction, which is a non-covalent interaction between cations and π electron-rich aromatic (organic) ring structures. “Plants contain a lot of organic, aromatic-ring structures, so studying the cation- π interactions between organic molecules and Fe cations may provide fresh insights into how plants adsorb and transform Fe elements,” Liang explains.
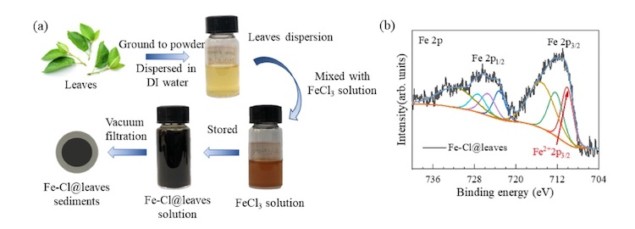
In their new study, the researchers used X-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FT-IR) spectroscopy and ultraviolet-visible (UV-vis) spectroscopy to observe the valence state of iron change from Fe3+ to Fe2+ in tea tree, wintersweet and other leaves. These observations confirmed that strong cation-π interactions take place between hydrated iron cations and substances that contain aromatic rings in the leaves. An additional theoretical study involving density functional calculations confirmed these findings and also revealed that the most stable adsorption sites for hydrated Fe3+ cations were located in regions with hydroxyl groups and aromatic structures.
The first electron counts – how anaerobic microbes ‘breathe’ iron
A highly efficient process
“The work provides an understanding of where soil-Fe2+ comes from, and how the Fe3+ is transferred to Fe2+ with the help of fallen leaves,” Liang tells Physics World. “This transfer mechanism is a highly efficient process and affords new insights into this valence change in plants.”
The researchers, who report their work in Chinese Physics Letters, observed that the transforming rate and the equilibrium constant of the Fe2+/ Fe3+ transformation might be strongly linked to the ambient temperature. They now plan to study the effect of temperature on the valence transformation in more detail. They also note that the acidity of the soil has a dramatic effect on the uptake of Fe2+ by plants, so they are planning measurements to quantify this effect.
“We are also interested in how the fallen leaves improve the quality of soil,” says Liang. “Ultimately, this could lead to strategies to increase crop production.”