Fast tracking of single molecules inside cells could provide important insights into their dynamic behaviour, such as the diffusion and underlying forces affecting their motion. However, achieving adequate imaging speed and precision has proved challenging. Now researcher’s spread across London, Oxford and Berlin have teamed up to address protein diffusion in neurons with a new type of super-fast interferometric nanoscopy.
The researchers proved a prevailing theory in the lab using the interferometric nanoscopy technique – that transmembrane proteins are in some sense confined in specific zones in the cylindrical axon of a neuron. This model would fit with the now almost canonical (yet difficult to investigate) theory first suggested by Akihiro Kusumi and colleagues in Japan and the US in their ‘picket fence’ membrane model, where the cytoskeleton has some role in corralling membrane proteins to elicit proper function and nanoclustering.
According to the picket fence model, cortical actin and transmembrane proteins in the membrane work together to create pockets of confinement. Proteins and lipids only exhibit free diffusion in this tiny zone – Kusumi describes the movement of the lipids to neighbouring corrals as ‘hop diffusion’. It is thought that this mechanism might provide a mechanism for fast spatial regulation of nanoclusters of proteins, aside from their phosphorylation state, association with other proteins or the cytoskeleton itself.
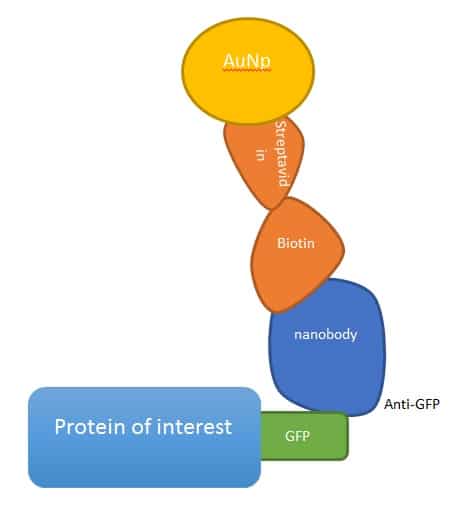
To investigate the theory, Gabi de Wit at the University of Oxford, UK, David Albrecht and Helge Ewers at King’s College London, UK, and the Free University in Berlin, Germany, used a technique, called interferometric scattering (iSCAT) with equipment put together by Philip Kukura at the University of Oxford. The approach combines light scattered from gold nanobeads (AuNPs) with reflected light from the glass–water interface of the microscope slide, which is usually cut out as background. The interference pattern detected is used to boost the signal, which manifests as a single point spread function that can be localised to nanometre precision in each frame. Combine this with acousto-optical beam deflectors, and iSCAT allows you to image at tens of kHz, fast enough to image the diffusive properties of transmembrane proteins in neurons. The speed of imaging is in practice faster than an average CMOS camera, meaning the scanned image appears constantly illuminated.
Applying iSCAT in neurons
To do the experiment they tagged three inert transmembrane proteins with nanobodies (tiny camel-sourced antibody fragments) bound to AuNPs, and tracked their movement with iSCAT. What they found was that in neurons, there were clear 180 to 200 nm segments in which proteins were free to move, which matched their prior work and the actin/spectrin rings seen in the axon initial segment.
In addition, they showed different diffusive models for all three of the membrane proteins surveyed. To back up the work, they also imaged thin epithelial cells from a common, well characterised cell line (U2OS) in the same system. Here, they confirm Kusumi-esque hop diffusion and the existence of corrals, where proteins halt their diffusive motion for up to 50 µs, before moving to the next nanoscale area. These behaviours were not present in the neuron.
Kusumi showcased his lab’s ability to track cell membrane lipids, to posit his picket fence model. This work now backs up the picket fence model, over the traditional Singer model of freely diffusing membrane proteins, and provides a window on a world that was simply too fast and small for us to see previously. In addition, this work emphasises nanoscale, and not just microscale location and diffusion as a likely regulatory mechanism for, at the very least, such membrane proteins. Past studies have suggested that receptor aggregation and swapping during virus entry occurs by using this kind of fast single particle tracking. Here, a new system is confirmed in neurons.
Full details can be found in the preprint on biorxiv.org.



