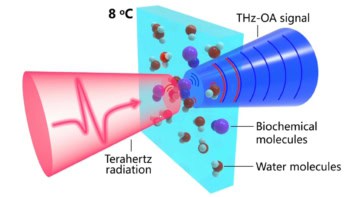
SCINTIX biology-guided radiotherapy (BgRT), performed on the RefleXion X1 linac, is a radiation delivery modality that uses real-time positron emission tomography (PET) to guide radiotherapy beamlets to radiolabelled tumours. Such guidance offers the potential to compensate for motion during treatments, reduce treatment volumes for moving tumours and increase intra-fraction treatment accuracy. In a first-in-human study, researchers in the US have now shown that SCINTIX dose distributions calculated from PET data were accurate and deliverable to lung or bone tumours.
Radiotherapy is often not considered an option for patients with multiple metastases, due to toxicity concerns and workflow issues relating to administering multiple treatments in a single session. In the future, PET-guided radiotherapy may be able to treat such patients by increasing delivery efficiency and reducing treatment margins related to motion and setup uncertainties.
The research team, headed up at Stanford Medicine and UT Southwestern Medical Center, identified a suitable dose of the radioactive tracer 18F-fluorodeoxyglucose (FDG) to perform SCINTIX therapy, and validated that the SCINTIX algorithm provides accurate dosimetry. The findings, reported in the International Journal of Radiation Oncology, Biology, Physics, support data submitted for US Food and Drug Administration (FDA) clearance of SCINTIX, obtained in 2023.
SCINTIX therapy uses a radiolabelled tumour as its own fiducial marker for targeting. BgRT technology differentiates itself from other types of real-time tumour tracking during radiotherapy by enabling the tumour to “communicate” its current position directly to the linac, using radiotracer uptake as a biological fiducial.
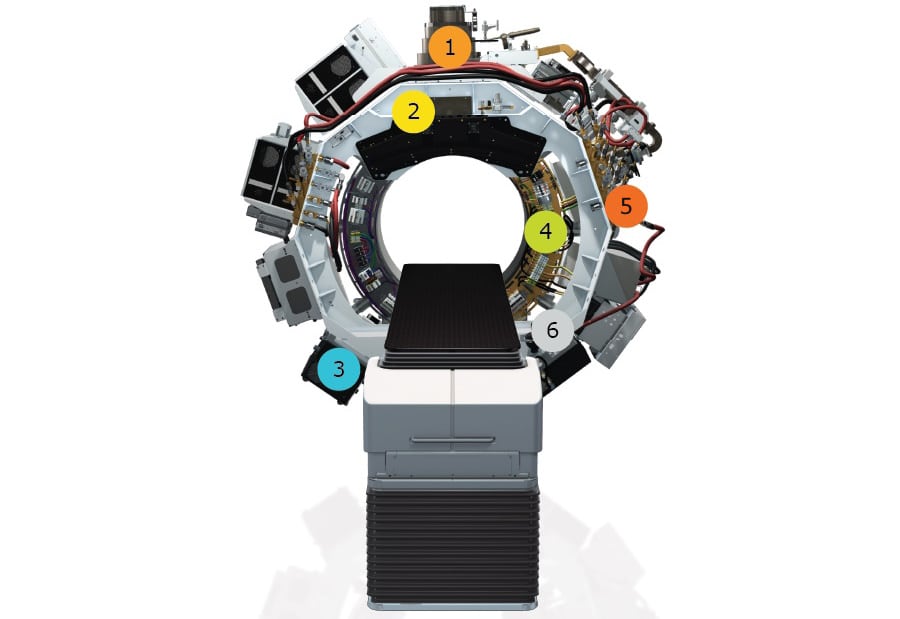
The RefleXion X1 is a hybrid imaging–therapy system that also delivers intensity-modulated radiotherapy, stereotactic body radiotherapy (SBRT) and stereotactic radiosurgery. The system’s integral PET detectors (comprising 64 scintillation multi pixel counter modules) are employed at three points in the BgRT workflow: to collect PET data from the patient for use in treatment planning; immediately prior to radiation delivery to evaluate whether the tumour’s PET avidity is consistent with the imaging-only session; and during BgRT delivery to guide the therapeutic beam.
Following CT imaging for initial patient set-up, real-time PET detects outgoing tumour emissions and delivers radiation beamlets to the tumour location with a response latency of 350–400 ms. The ability to conform radiation delivery to a moving tumour results in a tracked dose distribution characterized by high dose falloff and reduced dose to normal tissues.
First-in-human study
The BIOGUIDE-X study, led by Lucas Vitzthum and Murat Surucu from Stanford, and Daniel Chang of the University of Michigan, included two sequential groups of participants, all of whom had at least one FDG-avid targetable tumour in the lung or bone. The study in cohort I confirmed that a 15 mCi injected dose of FDG provided an adequate activity concentration (median of 5 kBq/ml) to enable SCINTIX therapy.
Using this dose, the researchers created emulated treatment fractions for cohort II – five patients with lung tumours and four with bone tumours. They used an emulated delivery technique in which PET data collected on the RefleXion X1 before the patient’s first and last SBRT fractions are converted offline to linac machine instructions and then “delivered” in silico to the patient’s CT anatomy. This generates an emulated dose distribution and dose–volume histogram (DVH) that can be compared with the approved treatment plan.
The researchers generated a SCINTIX treatment plan that met all clinical criteria for all nine participants. The activity concentration, normalized target signal and predicted DVH requirements at the PET pre-scan evaluation were met in 17 of the 18 emulated deliveries. Sixteen of the 17 evaluable emulated deliveries resulted in SCINTIX dose distributions that were comparable to the physician-approved treatment plan; the other was just below the 95% threshold for accuracy. All of the dose distributions, regardless of tumour location or SBRT fraction, were physically deliverable by the machine hardware.

Biology-guided radiotherapy system spares critical organs
“By mapping out the clinical workflow for SCINTIX BgRT and studying the emulated SCINTIX administration for patients at both the outset and conclusion of their SBRT regimen, we’ve gained valuable insights into how the RefleXion system would respond to each patient’s PET emissions through radiotherapy,” says Surucu.
Vitzthum tells Physics World that the next steps “include evaluating the performance of SCINTIX for tumours outside of the bone and lung. These sites include lymph nodes and soft-tissue metastases such as liver or adrenal metastases. We are also evaluating SCINTIX with alternate radiotracers, including prostate-specific membrane antigen.”