What occurs within cells is well documented as being important to both healthy function and disease, but what occurs outside of the cell can be just as important – if not more so – to these same functions. The research field of “mechanotransduction” concerns the effect of physical interactions between a cell and its extracellular environment (the extracellular matrix), and how these interactions affect cell behaviour and expression. Modelling these interactions in the lab is vital to discovering their roles in both physiological and pathogenic processes.
For tissue engineering and biofabrication, researchers use specialized biomaterials called hydrogels to replicate the native extracellular matrix of a cell. Typically, hydrogels are composed of polymers that allow for high water saturation and have tuneable physical properties, making them important tools for mechanotransduction research.
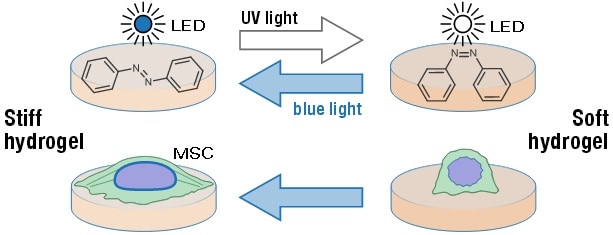
Researchers from the University of Manchester have developed a sophisticated hydrogel system with reversibly tuneable physical stiffness, controlled by either near-UV or blue light exposure. This development allows for the stiffness of a cell’s external environment to be controlled by irradiating the hydrogel with specific wavelengths of light (ACS Appl. Mater. Interfaces 10 7765).
This technique builds on previous methods of tuning hydrogel stiffness, which commonly rely on either altering pH or ionic concentration, or chemically modifying the hydrogel. All of these mechanisms have the potential to alter cell behaviour and adversely affect results beyond those variables intended to be measured.
The hydrogel system developed by the Manchester team uses polyacrylamide polymers with azobenzene cross linker, and negates the need for cell behaviour-altering treatments. The researchers demonstrated the capability of the hydrogel system to reversibly alter stiffness, by reducing hydrogel stiffness through exposure to near-UV light, and heightening its stiffness through exposure to blue light.
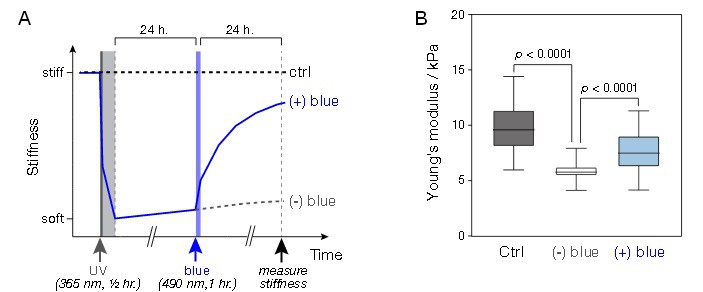
To decipher the effects of this approach on cell viability and DNA damage, the group used their hydrogel system in conjunction with mesenchymal stem cells. This class of stem cell is used commonly in tissue engineering and regenerative medicine studies, owing to its capability to have growth directed by being placed in substrates of specific stiffness. The researchers established that neither the blue light nor UV light was able to cause significant cell death, although it was clear that UV light significantly damaged cellular DNA. This finding led the group to implement cell seeding onto the hydrogel system after UV irradiation.
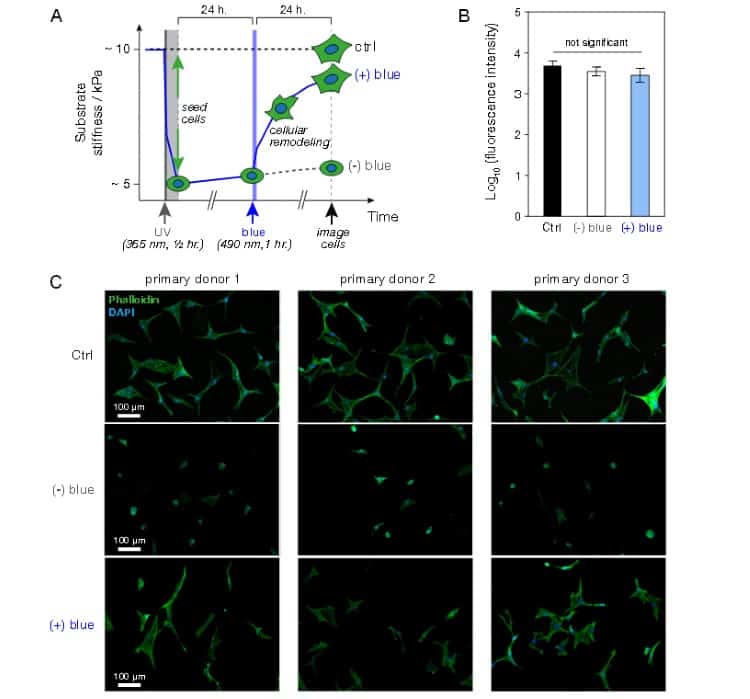
The team also investigated specific effects on cell morphology from the altered stiffness. They found that the physical stiffening of the hydrogel promoted cell spreading, whereas the softer UV-treated hydrogels had a more rounded morphology. The authors state that these results reinforce previous literature findings.
The hydrogel system developed by these researchers allows analysis of substrate stiffness and its effect on cell behaviour in real time. The authors indicate that future biological applications for this system include studying mechanotransduction in relation to fibrosis, aging and even developmental biology.



