Physicists in the Netherlands say they have explained the mystery of why tiny nanobubbles on wet surfaces can endure for weeks, despite having extremely high internal pressures. Measuring about 1 µm across and 20 nm high, these highly stable entities are tiny versions of the ordinary bubbles that cling to the inside of a full beer or champagne glass. According to James Seddon and colleagues at the University of Twente, nanobubbles last for so long because gas molecules inside them do not escape into the main liquid, but instead hitch a ride on a circular path that puts them back inside.
Unlike the air in larger bubbles, which is just slightly above atmospheric pressure, physicists know that nanobubbles have internal pressures of tens or even hundreds of atmospheres. At such pressures, the conventional model of diffusion suggests that all the gas inside these tiny bubbles should be absorbed by the liquid in a few microseconds. So when nanobubbles were first spotted a decade ago, physicists were left puzzled by the fact that they hang around for weeks.
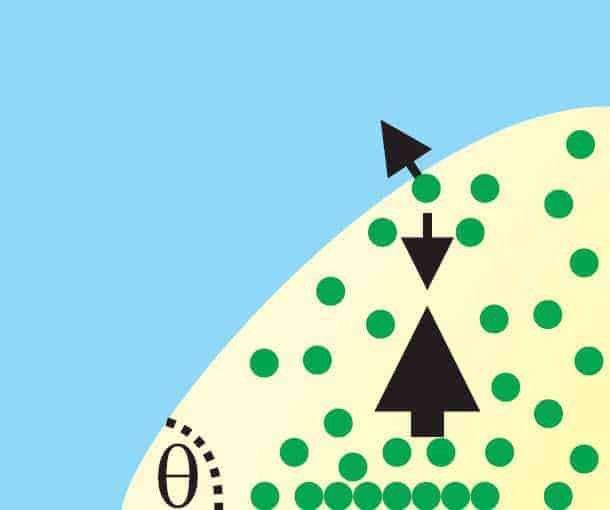
But the Seddon group’s new calculations and experiments could explain why. Its theory relies on two important physical properties of the system. One is that the nanobubbles are so small that a gas molecule will usually travel from one side of a bubble to the other without colliding with any other gas molecule. The other is that a gas molecule sticking to the surface inside the bubble is most likely to leave the surface in the perpendicular direction.
Flowing fountain
According to Seddon’s team, what happens first is that such gas molecules move away from the solid surface and towards the liquid interface of the bubble. But because they do not collide along the way, all the molecules are moving in approximately the same direction when they strike the edge of the bubble. This imparts a momentum to the liquid at the interface, causing liquid to flow along the bubble away from the solid surface.
The gas molecules get caught up in this flow and are swept towards the apex of the bubble. At this point the flow leaves the bubble and loops back down to the solid surface, bringing the gas molecules with it – and creating a fountain-like effect. But instead of going with the flow back along the bubble, the gas molecules tend to stick to the surface and move into the bubble. Here they are released from the surface and the process repeats itself.
To test its theory, the researchers used an atomic-force microscope to look for the outward flow at the apex of nanobubbles. Seddon told physicsworld.com that they measured an upwards force of about 1.3 nN above their nanobubbles. This is in line with their theory, which predicts a force of about 1 nN.
Bursting their bubble?
However, not all physicists agree with the team’s conclusions. Phil Attard of the University of Sydney – who pioneered the study of nanobubbles – told physicsworld.com that he finds fault with the thermodynamics of the theory. “In my opinion the proposed model by Seddon and colleagues is not viable,” he says. The team now plans to confirm its findings by taking snapshots of the process by adding nanoparticles to the liquid, which should get caught up in the flow.
Gaining a better understanding of the physics of nanobubbles is important, according to Seddon, because their existence is a fundamental problem of fluid dynamics. Nanobubbles also have a number of technological applications. They could, for example, play an important role in microfluidics, where their presence on the walls of tiny channels could make it much easier for fluid to flow.
The research is described in Phys. Rev. Lett. 107 16101.



