An organ-on-a-chip is a microfluidic device with continuously perfused chambers in which cells are cultured to obtain functional units that mimic organ functions. The organ-on-a-chip stands out as a model for investigating the basic mechanisms of physiology and disease.
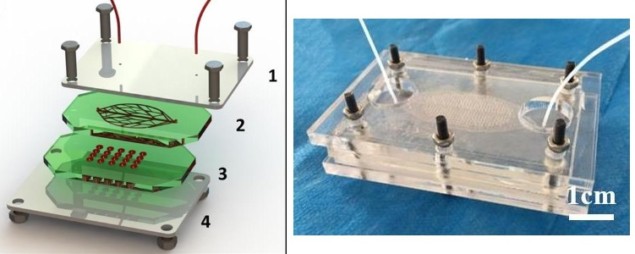
Since the design of the microfluidic system is key to better control of fluid flow through the organ, a team from Stanford University and Xi’an Jiaotong University have introduced a novel, leaf-templated, microwell-integrated, microfluidic chip. The chip combines a leaf venation layer (the lines on the leaf are called “leaf veins” and are responsible for water, nutrient and sugar transport, together with biomechanical support and protection) for fluent fluid flow, and a microwell-array layer for cells to reside (Biofabrication 10 025008). This leaf-templated design provides a novel tool for high-throughput cell experiments.
When envisioning a novel strategy for developing a microfluidic chip, the authors of this study were inspired by nature. More specifically, they imagined that leaf venation, which represents a hierarchical network involved in flow throughout the entire leaf, could be used as a vascular system for cell experiments. This report is the first to introduce a chip with a leaf as a template.
Device design
The leaf chip is made up of a PDMS (polydimethylsiloxane, the most widely used silicon-based organic polymer) layer with leaf-templated microfluidic channels fabricated through replication of a leaf venation skeleton, and another layer containing microwell arrays, fabricated through PDMS casting on a 3D printed resin mould. These layers are assembled to form the pathway that provides a flowing culture medium for the microwells in which cells are grown.
The microwells are connected to the leaf-templated microfluidic channels so that the cells can receive nutrition and oxygen. The structure of the device was analysed using specialized software, which allows the extraction of statistics concerning the dimension, position and connectivity of all veins in the network.
Testing the device
First, by using a solution of nanobeads, the authors tested the perfusion of medium flowing into the microwells, and confirmed that the culture medium is perfused throughout the entire leaf venation network. To verify the feasibility of the device, they inoculated cells in the microwell arrays and cultured them in a perfusion platform consisting of a syringe pump and a medium reservoir. The authors noticed that the microwells were uniformly seeded with cells and that after two days of perfusion, cells were viable and grew.

The results of this study suggest that the leaf-templated venation microfluidic chip is capable of supporting cell growth, thus offering a novel method for the fabrication of microfluidic chips that meet the requirements of high-throughput experiments and have applications in pharmacological studies.



