The inexorable rise of MRI in radiotherapy treatment planning shows no sign of stalling. Superior soft-tissue contrast (versus CT scanning) and the ability to visualize a rich matrix of functional information – including diffusion processes, blood volume and oxygenation, and localized metabolic activity within tumour sites – represent a winning combination for medical physicists and radiation oncology teams. Equally compelling is the fact that MRI interrogates the patient using non-ionizing radio waves, a major plus when treating children and in cases where repeat imaging scans are needed to track tumour response to radiation treatment.
Current standard-of-care means that MRI is used in tandem with a CT scan for treatment planning, with the patient ideally imaged in the treatment position. The fused CT-MRI dataset provides the MR information needed to outline the tumour volume and organs at risk, while the CT is used for dose calculation. However, the devil, as always, is in the detail – and it’s that detail that matters to CIRS, a US manufacturer of tissue-equivalent phantoms and simulators for medical imaging, radiation therapy and procedural training.
We’re trying to ensure that the geometric fidelity of the MR image is the best we can get when it’s incorporated into radiotherapy treatment planning
Niranjan Venugopal, CancerCare Manitoba, Winnipeg, Canada
At the upcoming American Association of Physicists in Medicine (AAPM) Annual Meeting in San Antonio, US, CIRS will feature a suite of dedicated products – including its MRI grid phantoms and proprietary Distortion Check software – designed to help medical physicists quantify and, in turn, track image distortion issues that can arise in MR images for radiotherapy treatment planning. Such distortion is unavoidable and has its origins in tiny perturbations in the uniformity of the MRI scanner’s magnetic field, caused by the patient and gradients used to image the patient.
Stereotactic QA
Among the early-adopters of the CIRS MRI grid phantoms was Lukáš Knybel, a biomedical engineer at University Hospital Ostrava in the Czech Republic, where’s he’s part of a five-person medical physics team looking after all aspects of treatment planning, dosimetry and QA in the radiation therapy department.
Knybel is particularly interested in quantifying the spatial distortion of MR images used in planning for stereotactic radiosurgery (SRS), a precision-targeted radiotherapy technique that has registered significant success in treating single and metastatic tumours in the brain. “More and more we are using MR images to make contours and prepare the SRS treatments – I wanted to know how precise that MR imaging can be,” he explains.
With this in mind, Knybel initiated a small-scale research study using the CIRS Model 603A SRS grid phantom and Distortion Check software. The 603A is designed specifically for SRS planning and comprises a tissue-equivalent, anthropomorphic design that can be imaged using X-ray, CT and a range of MRI sequences.
“The biggest benefit of this phantom is that you can check the MR image distortion by yourself, without having to rely on the regular machine service carried out by the MR equipment vendor’s engineering team,” he explains. Using the 603A, Knybel can check distortion in the clinic’s MR imaging sequences – and can do that quickly and easily each time the MR scanner is recalibrated and serviced by the manufacturer. “It gives us a QA double-check after the servicing – to be totally sure that we are using high-quality MR images with low distortion,” he adds.
The 603A and Distortion Check are now a fundamental part of Ostrava’s QA workflow for SRS, says Knybel. “Before we had this phantom, we had to trust in the accuracy of the MR images generated by the radiology department. Now we have an added degree of reassurance for the treatment team.”
Knybel cites the powerful visualization capabilities of Distortion Check as a significant benefit, enabling clinical users to quickly identify the biggest areas of spatial distortion in an MR image. In a typical SRS treatment scenario, for example, the region around the cochlea (part of the inner ear) is prone to significant image distortion. “Because of this we’ve changed our QA workflow to target the MR imaging isocentre as closely as possible to the tumour or area of interest, while adjusting our treatment margins to take account of distortion errors,” he explains.
Large-field QA
While Knybel and his colleagues in Ostrava are focused on minimizing the impact of MR image distortion in SRS planning, others are using CIRS’s large-field grid phantom (Model 604) as part of their QA for whole-body MRI in radiotherapy treatment planning. A case in point is Niranjan Venugopal, a medical physicist in the department of radiotherapy physics at CancerCare Manitoba in Winnipeg, Canada.
“MRI is like the ‘golden child’ of imaging – it has within it many capabilities to contribute to the continued improvement of radiotherapy,” Venugopal explains. “However, before we bring any technology into the clinical environment we have to make sure it’s safe and not introducing any unknown errors. If MRI sequences are not optimized they can potentially be a source of error in the treatment plan.”
The work that Venugopal and colleagues are doing with the Model 604 and Distortion Check is to ensure that MRI is deployed robustly in a radiotherapy context. Their goal: to maximize clinical effectiveness in terms of the precision targeting of diseased tissue.
“We’re trying to ensure that the geometric fidelity of the MR image is the best we can get when it’s incorporated into radiotherapy treatment planning,” he notes. “The CIRS solutions allow us to implement a systematic approach to QA of MR spatial distortion with very little effort.”
That QA begins with the commissioning of a new MR scanner and characterization of the machine’s baseline imaging performance versus manufacturer specifications. “A little bit of MR image distortion is forgiven in traditional radiology,” says Venugopal, “but in radiotherapy spatial distortion is unforgiving because it doesn’t allow us to correctly localize the tumour or fuse the MRI data to the planning CT.”
This is where the Model 604 phantom from CIRS comes into its own – and not just during machine commissioning, also for regular QA of MR image distortion over the lifetime of the MRI scanner. “The phantom allows you to make spatial distortion measurements,” says Venugopal, “but the Distortion Check software enables you to track changes in spatial distortion over time.”
Distortion vs time
Venugopal, for his part, was among the CIRS customers involved in beta-testing of Distortion Check prior to the software’s commercial release. Used in conjunction with the CIRS MRI grid phantoms, the software allows users to acquire and upload MR images of their phantom to an online repository.
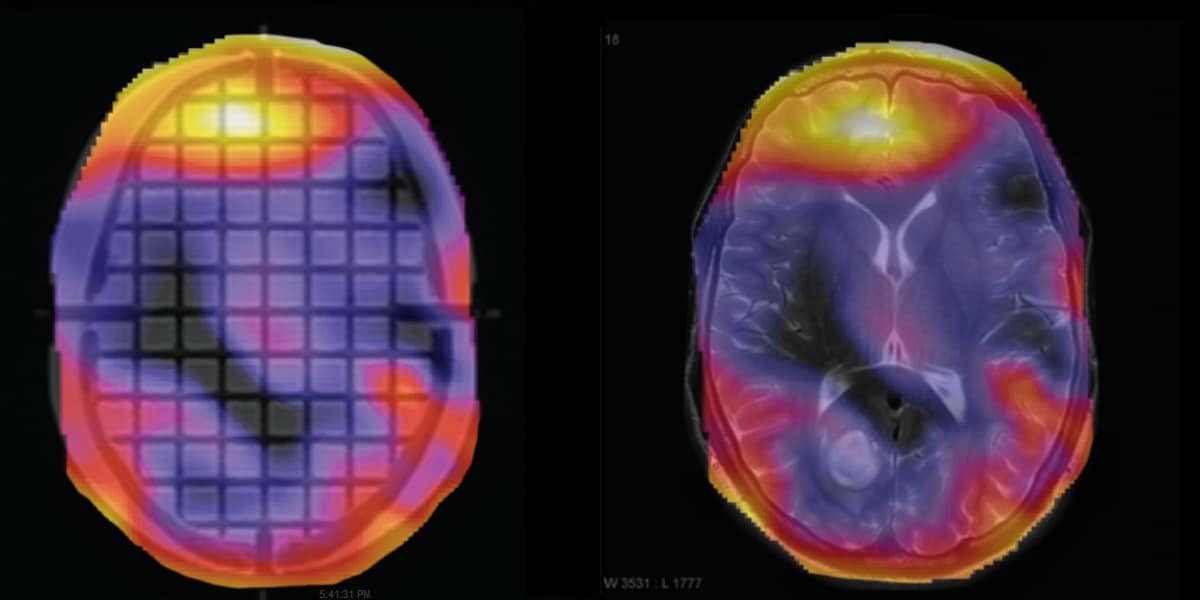
After detecting all grid intersections in the MRI phantom, the software compares the 3D grid points to a template or “ground truth”. Outputs include axial, sagittal and coronal views of the image distortion as well as a 3D scatter plot to view the data.
If needed, MATLAB format files containing detected grid intersections and registered CAD references can be downloaded to analyse the results in more complex algorithms. To further help take meaningful decisions, it is possible to generate an overlay of the distortion magnitude, which can be used with the phantom scan or with a patient’s scan.
“The analysis allows you to compare your current MR image versus the ground truth,” adds Venugopal. “Moreover, Distortion Check saves the distortion data over time so that users can track changes and establish thresholds for image distortion over multiple MR scanning techniques.”
- CIRS will be exhibiting on booth #1006 at the American Association of Physicists in Medicine (AAPM) Annual Meeting in San Antonio, Texas, from 14-18 July 2019.
MRI phantoms: a closer look
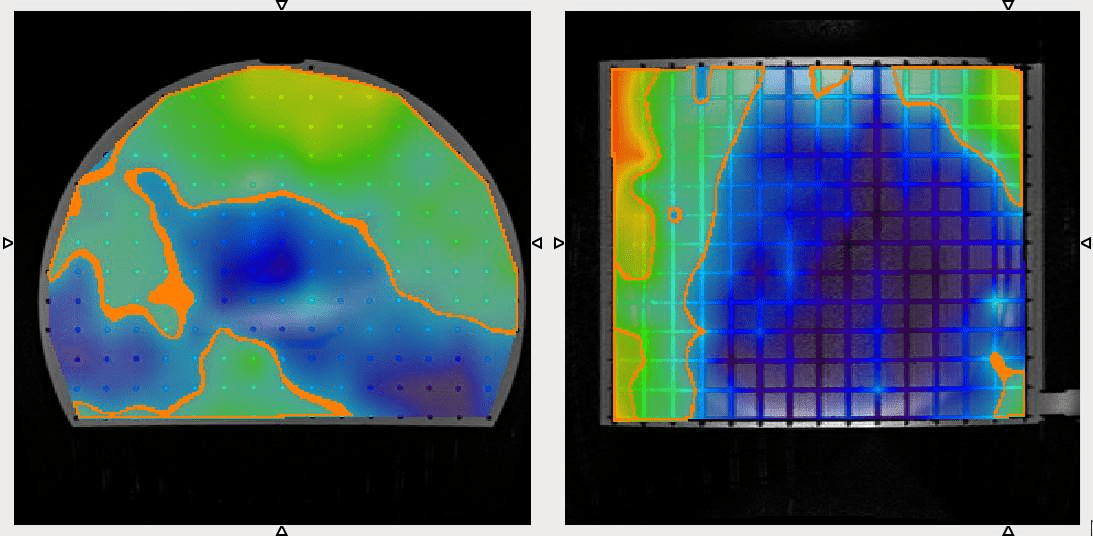
The CIRS Model 603A is a tissue-equivalent anthropomorphic phantom designed specifically for the assessment of MR image distortion in SRS planning (below). The entire intracranial portion of the skull volume is filled with an orthogonal 3D grid of 3 mm diameter rods spaced 15 mm apart. Five extended-axis rods intersect at the reference origin of the grid. The end of each extended axis is fitted with CT/MR markers allowing for accurate positioning with lasers and coregistration of CT and MR image sets. The 603A can also be used in CT/MR image-fusion studies. (Courtesy: CIRS)
The CIRS Model 604 is a large-field grid phantom for whole-body MR distortion QA (below). Comprising a leak-proof PMMA cylinder (330 mm diameter, 300 mm long), the Model 604 is filled with an orthogonal 3D grid of 3 mm diameter rods (spaced 20 mm apart) to provide complete geometric data throughout the imaging volume. The phantom is marked for ease of alignment to positioning lasers and is designed for use with both curved and flat gantry tables. (Courtesy: CIRS)