Humans have been making glass for thousands of years and have benefited from its many useful properties. However, understanding the glassy state — a solid-like state in which the atoms are in irregular positions much like atoms in a liquid — is one of the great unsolved mysteries in condensed-matter physics.
Now, researchers in the UK, Japan and Australia have shed new light on this old problem by studying how atoms in glass could become “jammed” into irregular arrangements when molten glass is cooled. Their results confirm predictions made some 50 years ago that the cooling atoms first join together to form icosahedral structures (20-sided objects), which then find it impossible to orient themselves into a crystalline lattice. The team believe that their insights into the formation of glass might lead to the creation of new materials, such as “metallic glasses”.
Physicists believe that glass is formed when a liquid is cooled and its constituent atoms are unable to arrange themselves in a stable crystalline state. Instead, the atoms become trapped in a state of “dynamical arrest” — the atoms can be likened to cars in a perpetual traffic jam that never reach their final destination of a crystalline structure. Understanding why this occurs has proven very difficult because the individual atoms are much too small to be tracked using optical microscopes — and electron microscopes cannot image the atoms in 3D.
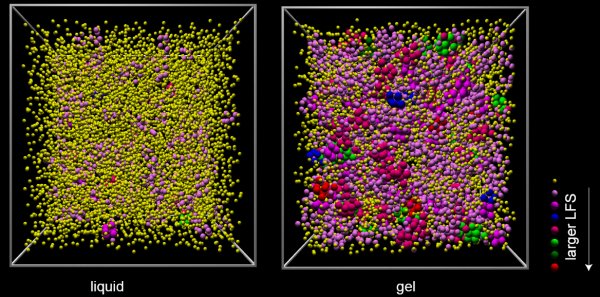
Dynamical arrest is also involved in the formation of many gels, which comprise micrometre-sized colloidal molecules that can be observed using a microscope. Now, Paddy Royall of the University of Bristol together with colleagues at the University of Tokyo and the Australian National University have studied such gels to gain an insight into how dynamical arrest could occur in glasses (Nature Materials doi: 10.1038/nmat2219).
Five-fold rotational symmetry
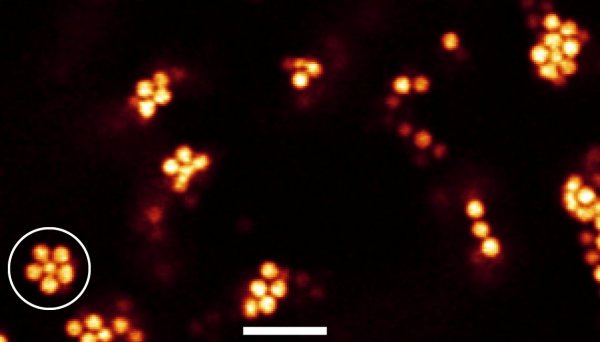
The researchers watched as a colloidal liquid cooled to become a gel and saw the formation of structures with five-fold rotational symmetry — a hallmark of icosahedral structures. Using computer analysis, they found that these structures became more numerous upon cooling to form jammed icosahedra-like structures.
Crystallization requires space to be filled by a repeating pattern of atoms or molecules — but the icosahedra seen by Royall and colleagues cannot fill space without leaving gaps. This is the 3D analogue of the fact that repeating 2D pattern cannot be made using five-sided tiles.
“These structures are not compatible with crystallization, which supports an idea going back to Sir Charles Frank, also from the University of Bristol,” Royall told physicsworld.com. “In the 1950s, he was the first to identify such low-energy structures and emphasized the five-fold symmetry of icosahedral arrangements of atoms.”
Royall believes that the team’s insights into dynamical arrest may even help make metallic glasses — metals with the same structure as glass. This is unlike coventional metals, which are crystalline and contain “grain boundaries” between regions that are perfect crystals.
Defects at these boundaries often cause the metal to fail under stress. Such boundaries would not occur in metallic glasses, making them less prone to failure. Metallic glasses might find use in a variety of products that need to be flexible, such as aircraft wings and engine parts.
The researchers are now expanding on their work to consider more examples of systems that undergo arrest. They will also apply their experimental and analysis techniques to study other major outstanding problems in condensed matter physics, and develop novel materials.