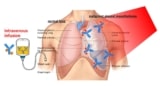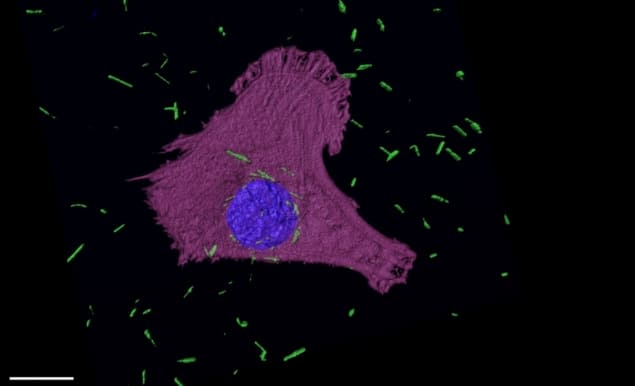
Gold nanotubes can destroy cancer cells, according to physicists and medical researchers at the University of Cambridge and the University of Leeds. They found that their nanotubes, which were tuned to have strong near-infrared absorption, can enter mesothelioma cells and destroy them when heated with laser light.
Every year, more than 2700 people in the UK are diagnosed with mesothelioma. This cancer usually grows in the pleural membrane, a thin lining that surrounds the lungs. The vast majority of cases are caused by exposure to asbestos dust. When damaged, asbestos releases microscopic fibres that can be inhaled. These fibres can then migrate through lung tissue into the pleural membrane and cause mesothelioma to develop. Asbestos has been banned in the UK since the late 1990s, but mesothelioma can take from 15 to 60 years to develop.
“Mesothelioma is one of the ‘hard-to-treat’ cancers, and the best we can offer people with existing treatments is a few months of extra survival,” says Arsalan Azad at the University of Cambridge. “There’s an important unmet need for new, effective treatments.”
To develop a potential treatment, the researchers turned to gold nanotubes. They hypothesized that if absorbed by mesothelioma cells and then heated using near-infrared light, these hollow tubes – one thousandth the width of a human hair – would destroy the cancer cells.
Tests in an aqueous solution showed that, when heated with a laser at a wavelength of 875 nm with a power density of 1.9 W/cm2, the nanotubes increased in temperature by up to 9°C, high enough to cause localized killing of cancer cells. Next, the team added the nanotubes to mesothelioma cell cultures and tracked them using various microscopy techniques, observing that the nanotubes were absorbed by the cells.
The researchers then exposed mesothelioma cell cultures with and without the gold nanotubes to the near-infrared light for 10 min. Laser irradiation alone did not cause cell death, but laser exposure combined with the nanotubes killed roughly half of the cells. They report their results in Small.
Key to the nanotubes’ potential to destroy cancers within the human body is their tunability. Stephen Evans, a physicist at the University of Leeds, says that there are two near-infrared windows in which light has good optical penetration through tissue. He explains that you need to tune the nanoparticles so that they best absorb – and convert to heat – light at those wavelengths.
The optical properties of gold nanomaterials are controlled by the density of free electrons that the light couples to and causes to oscillate, Evans explains. “That is dependent on the size, in our case, the thickness of the wall of the gold,” he says. “If we made thicker walled gold, we would shift the absorbance wavelength, and if we made the walls thinner, we would shift it in the opposite direction.”
The nanotubes are created using a solution-based technique. First, a silver seed particle is grown into a silver nanowire, and then a gold salt is added to the water-based solution. The gold deposits onto the surface of the silver, which oxidizes and becomes water soluble. The silver then dissolves away leaving the gold nanotube.
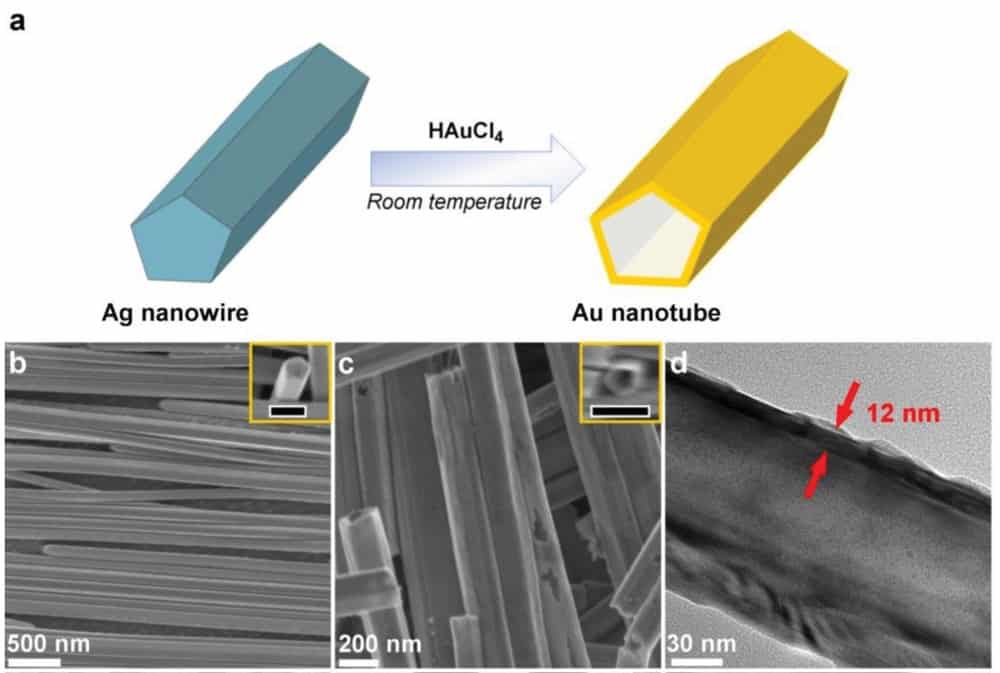
Evans tells Physics World that the thickness of the gold is tuned by controlling the size of the silver nanowires. In the study, the silver nanowires were around 100 nm in diameter, which led to a gold thickness of 12 nm. The team also used nanowires with average diameters of 65 and 193 nm as templates to prepare gold nanotubes with thinner and thicker walls. “We showed that we could make silver nanowires of different diameters and thereby produce gold tubes with different wall thicknesses,” Evans says.
Brighter prospects for treating a rare lung cancer
Part of the attraction of using these gold nanoparticles to treat mesothelioma is that they have similar dimensions to asbestos fibres. The hope is that that they will also get trapped in the same part of the body, aiding the delivery of treatment. “We are sort of mimicking what causes the disease so we can use that as a treatment,” Evans explains.