Borophene – a sheet of boron just one atom thick – can be stabilized in air by bonding its atoms with hydrogen, researchers in the US have discovered. The new technique was developed Mark Hersam at Northwestern University and colleagues, who found that hydrogenated sheets of borophene (called borophane) oxidized far more slowly in air than pure boron sheets. Their approach could enable researchers to finally realize many of the proposed applications of borophene – which were previously seen as impractical outside the lab.
In its atomically thin 2D form, boron has a diverse array of crystal lattice structures. Together named borophene, these sheets have many desirable properties: including high mechanical strength, flexibility, and phonon-mediated superconductivity. Like carbon-based graphene, these 2D materials hold the potential to revolutionize many aspects of electronics. However, unlike graphene, borophene is much trickier to fabricate into practical devices.
While graphene can be produced by simply peeling away layers of graphite, borophene must be synthesized directly on a substrate: a process first demonstrated by Hersam and colleagues in 2015. Unlike graphene, borophene rapidly oxidizes when exposed to air, removing its conductivity. This means that any experiments on the material must be carried out in ultra-high vacuum conditions, severely restricting the integration of borophene within practical devices.
Chemical functionalization
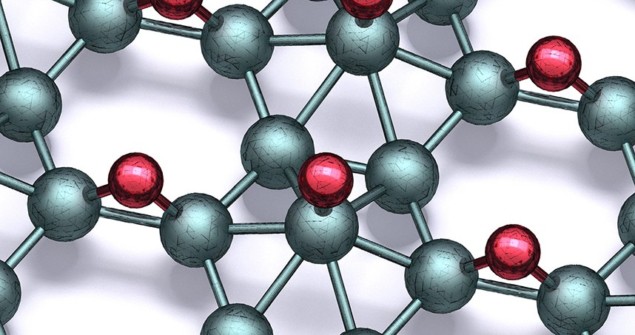
Previously, chemical functionalization by adding different atoms has been widely used to fine-tune the electronic properties of materials including graphene. Among the resulting products is graphane, in which the carbon atoms are bonded with hydrogen. Inspired by this process, Hersam’s team exposed borophene to atomic hydrogen in ultra-high vacuum to produce sheets of borophane featuring boron atoms bonded to hydrogen in several different ways.
Graphene-like boron made for the first time, claim researchers
The researchers then used a combination of atomic-scale imaging, spectroscopy, and theoretical calculations to determine the diversity of crystal lattice structures of their new material. Overall, they identified eight distinctive bonding patterns, each of which retained the desirable traits of borophene. The team also showed that their process could be entirely reversed through the thermal desorption of hydrogen – returning the boron to its original pure state.
Outside the vacuum chamber , Hersam and colleagues found that the oxidation rate of borophane was two orders of magnitude lower than borophene – demonstrating a far higher stability in air. This resilience at standard temperatures and air pressures could now significantly improve the prospects for the practical use of atomically-thin boron outside the lab. Applications could include batteries, sensors, solar panels, and quantum computers. If achieved, the team predicts a potential revolution in electronics; comparable even with previous advances brought about by graphene.
The research is described in Science.