Membranes made from graphene oxide could act as perfect molecular sieves when immersed in water, blocking all molecules or ions with a hydrated size larger than 9 Å. This new result, from researchers at the University of Manchester in the UK, means that the laminated nanostructures might be ideal for water filtration and desalination applications.
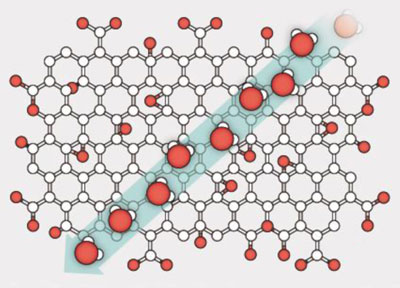
Graphene is a sheet of carbon just one atom thick in which the atoms are arranged in a honeycomb lattice. Graphene oxide is like ordinary graphene but is covered with molecules such as hydroxyl groups. Graphene-oxide sheets can easily be stacked on top of each other to form extremely thin but mechanically strong membranes. These membranes consist of millions of small flakes of graphene oxide with nanosized empty channels (or capillaries) between the flakes.
Two years ago, a team of researchers led by Andre Geim – who was first to isloate graphene in 2004 – found that graphene-oxide membranes were impermeable to all gases and vapours except for water. In fact, Geim and colleagues found that water passes through a film of graphene oxide extremely fast, while all other gases and liquids are blocked by the film. Even helium, which is extremely difficult to block, cannot pass through the membranes – but water vapour goes through so quickly that it is as if the membranes are not even there. This happens because the graphene-oxide sheets are arranged in such a way that there is room for only one layer of water molecules. In the absence of water, however, the capillaries shrink and do not let anything through this way, thus making the material impermeable to everything but water.
Now, Geim’s team has found that when the membranes are immersed in water, as opposed to just being exposed to water vapour or ambient humidity, they appear to swell slightly and are able to block all molecules or ions with a hydrated size larger than 9 Å. (A hydrated sugar molecule, for example, has a diameter of 10 Å.) What is more, the membranes are able to distinguish between atomic species that differ in size by only a few per cent. In addition, ions that are smaller than 9 Å across can pass through the membranes 1000 times faster than is expected by simple diffusion processes alone.
“Ion sponging”
“We believe that this last phenomenon is thanks to another exceptional property of graphene-oxide membranes that we have called ‘ion sponging’,” says co-team-leader Rahul Nair. “The capillaries between the individual graphene-oxide flakes appear to act rather like powerful little vacuum cleaners that ‘suck up’ small ions.”
The Manchester researchers produced their membranes by stacking thousands of individual layers of graphene oxide on top of each other using simple techniques such as vacuum filtration and spray-coating. The graphene-oxide sheets are separated from each other by about 6–7 Å when dry; but when the sheets are immersed in water, this separation increases to about 11–12 Å, because not one but two layers of water are lodged between the sheets. “Water layers formed inside this ultra-narrow graphene capillary can move very freely, something that helps ions and molecules that are smaller than the size of the capillary itself to pass through,” explains Nair.
Decontamination and desalination applications
According to the team, the membranes could be ideal for removing valuable salts and molecules from contaminated larger molecules – for example during oil spills. “More importantly, our work shows that if we were able to further control the capillary size below 9 Å, we should be able to use these membranes to filter and desalinate water,” says Nair.
Indeed, the team says that it is now busy looking at ways to control the mesh size of the graphene oxide and reduce it to about 6 Å so that the membranes can filter out even the smallest salts in sea water. “We might achieve this by preventing the graphene-oxide laminates from swelling when they are placed in water,” says Nair.
“Our ultimate goal would be to make a filter device from the carbon-based material that allows you to obtain a glass of drinkable water from sea water using a hand-held mechanical pump,” adds team member Irina Grigorieva.
Take a look at the video below, filmed at the University of Manchester lab, where the researchers talk more about the idea of using graphene to produce drinking water.
The current work is detailed in Science.
- This article first appeared on nanotechweb.org