Researchers at Yale University have reported a new imaging modality, known as deuterium metabolic imaging (DMI), in which sugars and other nutrients are labelled with a heavy hydrogen (2H) atom and subsequently administered to track and monitor their uptake and metabolism (Science Advances 4 eaat7314).
Mapping glucose metabolism is important for monitoring the development and treatment of cancer, as tumours metabolize glucose both at an elevated rate and through a different set of chemical reactions compared with healthy tissue — a phenomenon known as the Warburg effect.
2H, a form of atomic hydrogen with one extra neutron, is harmless and exists naturally in a very small abundance. In comparison, current methods of imaging glucose metabolism in the clinic rely on radioactive labelling of glucose molecules for detection by PET scans. In addition to the radiation risk, PET is unable to track the downstream metabolites of glucose and often gives misleading results in organs with intrinsically high glucose metabolism, such as the brain and the liver.
The researchers knew that 2H generated a signal in nuclear magnetic resonance (NMR) spectroscopy. This gave them the idea that 2H might also function as a metabolism tracer in traditional MRI scans.
“2H-labelled substrates are used in metabolic research in humans, but not combined with imaging. Our interest was piqued when we saw that 2H-labelled metabolites originating from 2H-labelled glucose could be detected in vivo at ultra-high magnetic field strengths,” explains first author Henk De Feyter. “Then it came down to trying this out at field strengths similar to those used in clinical MRI scanners. After those experiments were successful, it was obvious that DMI has great potential to become a widespread metabolic imaging tool.”
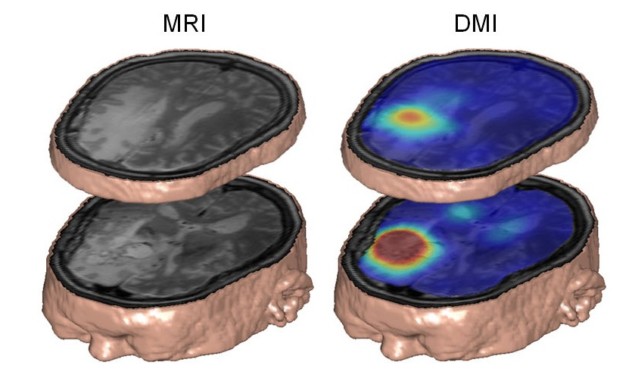
De Feyter, Robin de Graaf and colleagues showed how the DMI technique revealed dramatic differences in glucose metabolism between brain and tumour tissues in a rat glioma. They then went on to detect similar metabolic distributions in human subjects with brain tumours and in healthy controls.
While MRI scanners are significantly less sensitive to 2H than to the single proton of hydrogen nuclei, this loss is in part offset by the spin characteristics of the 2H nucleus. A DMI scan of a human patient with a brain tumour who drank 2H-labelled glucose dissolved in water revealed that the tumour was well contrasted to background healthy brain tissue, due to the Warburg effect.
The versatility of the DMI technique is also not limited to the brain, as the researchers were able to efficiently detect deuterium-labelled sugars stored as glycogen in both rat and human livers.
De Feyter and his team’s work showcases an exciting new clinical prospect for monitoring tumour metabolism in a non-invasive, patient-friendly way. This may have far reaching consequences for the development of novel cancer therapies based on the interruption of cancer cell metabolism.



