Tumour hypoxia is known to adversely affect outcomes for patients undergoing conventional radiotherapy. However, there are no published studies examining the influence of hypoxia on clinical outcomes for stereotactic body radiation therapy (SBRT), in which high doses of radiation are delivered in up to five fractions.
Hypoxia may have more of an impact in SBRT due to the fewer number of treatment fractions, which reduces the opportunity for re-oxygenation of hypoxic tumour cells. To investigate this premise, researchers from Yale University School of Medicine have used 18F-FMISO PET to quantify changes in tumour hypoxia in response to SBRT (Int. J. Radiat. Oncol. Biol. Phys. 102 174).
“There are two issues at play here,” explains senior author David J Carlson. “The hypoxic fraction of the tumour may be more important in determining treatment response in SBRT. We also show that high single doses of radiation can even increase the hypoxic tumour volume.”
Pilot study
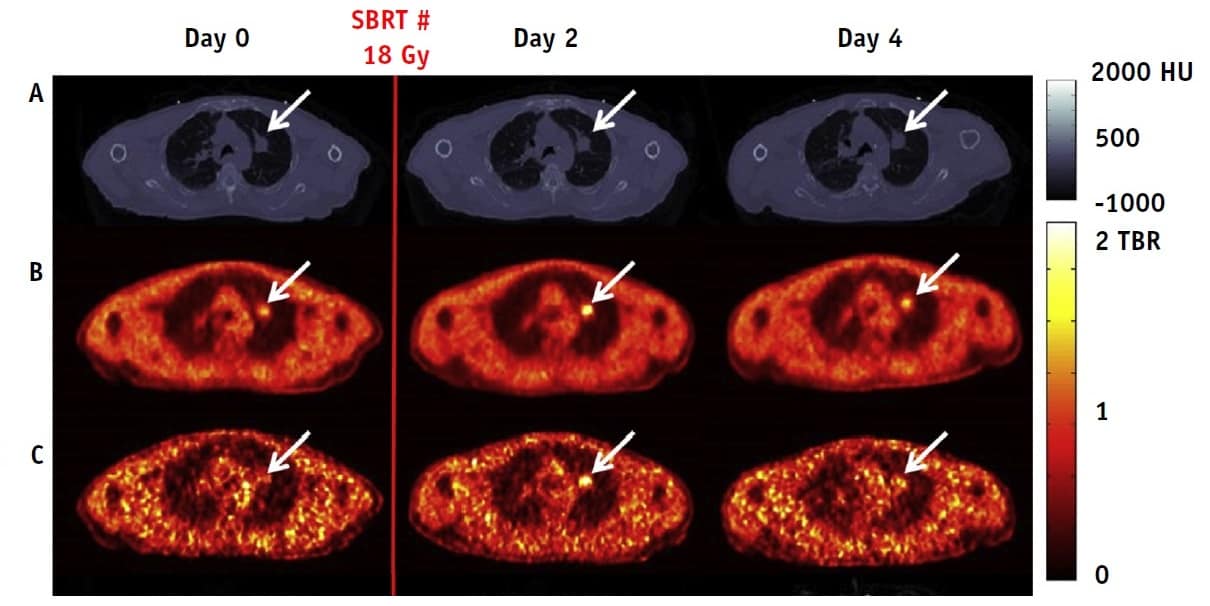
Carlson and colleagues examined six patients with early-stage non-small cell lung cancer (NSCLC) who received SBRT (three 18 Gy or five 10 Gy fractions) as standard of care. For each patient, the researchers recorded dynamic PET images at 0-120 min, 150-180 min and 210-240 min after injecting 18F-FMISO, which selectively binds to hypoxic cells. They performed this scan series immediately before, and two and four days after, the first SBRT fraction. After the final PET scan, they recorded a respiratory-gated 4D-CT.

For each time point, the researchers quantified the tumour hypoxic volume (HV): the ratio of hypoxic voxels, based on 18F-FMISO imaging, to total tumour voxels, based on a pre-treatment CT. Using end-expiration gate summed 210-240 min PET data, they calculated HV by assigning voxels with a tumour-to-blood ratio (TBR) of 1.2 or above as hypoxic.
Three of the five patients who completed the imaging protocol had detectable baseline tumour hypoxia, with baseline HVs of 23.5, 16.6 and 21.7% for patients 2, 5, and 6, respectively. In these patients, tumour HV increased (by up to a factor of 2.7) after SBRT delivery, to 40.4, 45.2 and 32.7% on day 2.
Between scans on days 2 and 4, the team observed a variable response, with tumour HV either decreasing to baseline level (patients 2 and 6) or remaining unchanged (patient 5). Patients 3 and 4 had no baseline hypoxia, and no increase after SBRT.
“Our preliminary clinical data suggest that hypoxic levels in tumours actually increase post-SBRT delivery, for patients with baseline detectable hypoxia,” says Carlson. “The clinical impact of this is still unclear, however, and we need larger trials to determine whether this phenomenon positively or negatively impacts tumour control. This is the first study to investigate the problem in human tumours and the role of vascular changes in treatment response is still unknown.”
Alternative approach
The team also used tracer kinetic parameters to calculate HV. Here, they used dynamic data (uncorrected for respiratory motion) to estimate the net rate of tracer influx (Ki) for each voxel, and quantified HV using a Ki threshold of above 0.0015 ml·min/cm3. Using this approach, baseline HVs were 20.6, 17.9 and 22.6%, for patients 2, 5 and 6, respectively.
In agreement with the TBR quantification trends, tracer kinetic analysis demonstrated an increase in mean HV between day 0 (20.3%) and day 2 (35.8%). The mean HV on day 4 increased to 41.2%, however, while TBR results suggested a decrease between days 2 and 4.

As to which calculation is more accurate, Carlson says that there is no simple answer yet. In theory, Ki-based HV estimates will ultimately be more accurate for quantifying hypoxia. In this work, however, the dynamic data used for kinetic analysis were not corrected for respiratory motion (while the static 210-240 min data used for TBR estimates were), which adds uncertainty to the Ki-based results.
In addition, explains first author Olivia J Kelada, “even if Ki is more accurate, it is currently impractical for routine clinical use because of the long dynamic scan times. It may be possible to shorten scan times, i.e. to collect data for the input function at an early time point and tumour uptake at a later time point; making this approach more feasible and comparable to clinical routine static PET scan times”.
“Larger studies with more patients are necessary to confirm our reported results,” says Carlson. “We would also like to further explore the impact of respiratory correction on tracer kinetic modelling results.”
Carlson notes that these results provide strong motivation for accounting for hypoxic volumes in clinical SBRT. For example, the SBRT delivery schedule for patients with more hypoxic tumours could be altered to increase the time between fractions. Another option is to add a therapeutic agent to target and exploit SBRT-induced hypoxia, such as a DNA repair inhibitor or hypoxic cell radiosensitizer. “This gives us another dimension on which we can optimize our radiotherapy treatments to improve patient outcomes,” he says.