By Hamish Johnston
Since the stricken Fukushima reactors in Japan started emitting radioactive materials a few weeks ago, physicists in places as disparate as San Francisco and Glasgow have been reporting elevated levels of radioactivity. Given the tiny quantities involved, just how are they detecting this material?
If you have access to the sort of kit that you might find in an undergraduate demonstration lab, you can do the measurement yourself. And a good guide to follow is a paper posted recently on the arXiv preprint server.
In the paper Eric Norman, Christopher Angell and Perry Chodash describe how they analysed rainwater samples from the San Francisco Bay area for traces of fallout from Fukushima – which is about 8000 km away.
From their base at the nuclear engineering department of the University of California, Berkeley, the team collected rainwater from three locations and brought it back to the lab. Each sample was placed in a Marinelli beaker, which is a special vessel for measuring the radioactivity of liquids.
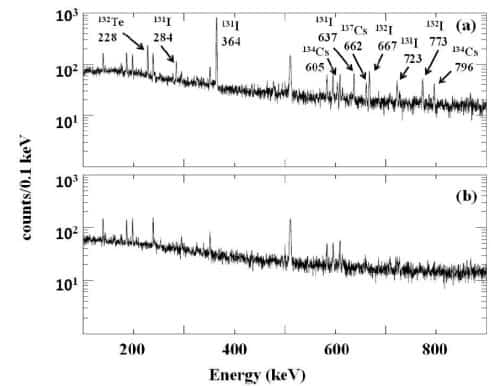
The beaker is placed over a high-purity germanium detector, which are used routinely to study gamma-ray spectra. Lead is used to shield the detector from background radiation. Gamma-ray spectra in the 0.02–1.58 MeV energy range are then collected for up to 24 hours. This range was chosen because it contains gamma rays emitted by radioactive isotopes of iodine, caesium and tellurium. These are produced by the fission of uranium and plutonium, both of which are used as fuel in the Fukushima reactors.
The team took its first samples overnight on 15 March and found no evidence for the three fission fragments. This allowed the team to set an upper limit on the concentration of iodine-131 in the samples of about 0.016 Becquerel per litre (Bq/l). The Becquerel is a unit of radioactivity pegged at one decay per second.
However, by 18 March evidence of radioactive materials began to emerge and the iodine-131 signal had risen to more than 5 Bq/l. The researchers also saw a corresponding rise in radioactivity associated with other isotopes of iodine, caesium and tellurium.
The top panel (above) is the gamma-ray spectrum of rainwater from 18 March, showing the presence of various isotopes. The bottom panel is the same spectrum of tap water.
The signal peaked on 24 March, with iodine-131 reaching 16 Bq/l. This is about four times the recommended level in drinking water. Radioactive iodine is dangerous because it concentrates in the thyroid gland. However, because of the relatively short half-life of iodine-131 (about 8 days), the Berkeley team doesn’t think that it will lead to significant exposure through drinking local water.



