Researchers are pushing magnetic resonance imaging to the nanoscale with the aim of developing a microscope that can directly image single molecules in 3D, explains Dan Rugar
A little over 30 years ago, the invention of the scanning tunnelling microscope (STM) revolutionized surface science and helped jumpstart the field of nanoscience. The STM and related tools such as the atomic force microscope allow researchers to quickly visualize individual atoms and molecules. However, they have one key weakness: they can only probe the top surface of an object. Is it possible to overcome this limitation with a microscope that looks below surfaces and directly images molecular structures in 3D at the atomic scale? This question underpins the emerging field of nanoscale magnetic resonance imaging, or nanoMRI – a technique that could represent a major breakthrough in microscopy and have a profound impact on some of the most pressing questions in structural biology.
The current gold standard for solving molecular structures is X-ray crystallography, in which an intense beam of X-rays is diffracted by atoms in a crystalline array of molecules. The diffraction pattern can be mathematically inverted to give the atomic structure of the molecules, unlocking the workings of fundamental biological units such as the ribosome and allowing pharmaceutical companies to develop more targeted drugs. However, X-ray crystallography can be applied only to those molecules that can be purified and crystallized, which represents a small fraction of biologically important structures. Nuclear magnetic resonance (NMR) spectroscopy is an alternative way to solve molecular structures, but it encounters difficulties for molecules above a certain size. Cryo-electron microscopy is also making strides in the pursuit of single-molecule imaging, but as with all forms of electron microscopy, radiation damage is a major issue.
Magnetic resonance imaging (MRI), a well-known technique in the medical arena, suggests an alternative approach. MRI is non-destructive, elementally selective and able to image below a surface in 3D. Like NMR spectroscopy, MRI relies on the detection of the weak magnetism associated with atomic nuclei, typically hydrogen nuclei (protons) in water and organic molecules. In the presence of a magnetic field, these nuclear spins precess at a certain frequency determined by the magnetic moment of the nucleus and the strength of the magnetic field. By imposing a gradient in the magnetic field, the precession frequency becomes spatially dependent, allowing images to be formed by analysing the frequencies of signals detected via an inductive receiver coil.
At first glance, the idea of extending MRI to the nanoscale seems preposterous. After all, nuclear magnetism is a notoriously weak effect because of the tiny value of the nuclear magnetic moment and the disorganized (paramagnetic) nature of the magnetism. For example, a single volume element in a medical MRI image typically requires at least 1018 nuclei to produce a detectable signal, resulting in a resolution in the millimetre to sub-millimetre range. In order to image molecular structure we require a resolution of 1 nm or better, necessitating a sensitivity improvement of at least 1016! Clearly great improvements in detection are required.
Feeling the force
The first serious proposal to extend MRI to the nanoscale came in 1991 from medical physicist John Sidles at the University of Washington. In a series of theoretical papers, Sidles outlined a method – now known as magnetic resonance force microscopy (MRFM) – for dramatically improving detection sensitivity based on the measurement of ultra-small magnetic forces. The technique exploits an effect every child learns at school: that two magnets either attract or repel one another depending on their orientation. In MRFM, the two magnets are a nanoscale ferromagnetic tip and the nuclear spins in the sample. By periodically flipping the orientation of nuclear spins in the sample using radio-frequency magnetic fields, the force between the tip and the sample nuclei is made to oscillate. The oscillating force is very weak, typically in the attonewton (10–18 N) range, and is detected by the slight vibration of a nanomechanical cantilever.
At the time of Sidles’ proposal, my group at IBM was working on new techniques in force microscopy and we agreed to test the MRFM idea. Since electron spins have a larger magnetic moment than nuclear spins, and thus provide a larger force signal, we decided to focus first on an electron spin-resonance experiment. After modifying one of our existing force-detection set-ups, I placed a microscopic crystal onto a small silicon-nitride cantilever and positioned it close to a small permanent magnet. To my delight, when I then turned on the radio-frequency magnetic field to periodically flip the spins in the sample, I was able to detect tiny oscillations of the cantilever caused by the flipping of the electron spins. Additional demonstration experiments at the micrometre scale followed quickly, including nuclear spin detection. As we continued to miniaturize the experiment, the field gradient from the permanent magnet increased, which allowed us to demonstrate 3D imaging of both electron-spin and nuclear-spin samples.
While micrometre-scale detection was fairly straightforward, extending the technique to the nanometre scale required a more serious effort. The key issue is the extremely small magnitude of the magnetic force generated by nanometre volumes of unpolarized spins, which requires a very small and sensitive cantilever with a low internal friction operating at low temperatures. In work carried out with John Mamin at IBM and students of Tom Kenny at Stanford University, we fabricated 100 nm thick, single-crystal silicon cantilevers that enabled us to achieve attonewton force sensitivity when operated at liquid-helium temperatures.
The advent of attonewton force sensing led to several key demonstrations of MRFM, with our group detecting a single electron spin in 2004. A few years later, we achieved an even more significant demonstration: 3D nanoscale MRI of a virus particle with a resolution better than 10 nm. Notable progress has also been made at several other institutions. Raffi Budakian and colleagues at the University of Illinois have pioneered the use of semiconductor nanowires for force detection, for instance, while Martino Poggio at the University of Basel has imaged multiple nuclear species within nanowires. At the Ohio State University, Chris Hammel and his students have done extensive work using MRFM to study ferromagnetic resonance in nanoscale magnetic objects, while John Marohn’s group at Cornell University has demonstrated ultrasensitive cantilevers with integrated magnetic tips that produce large field gradients.
Diamond alternative
A second detection technique for nanoMRI has recently emerged, based not on cantilevers but on a well-known atomic defect in diamond called a nitrogen vacancy (NV) centre. Here, substituting a nitrogen atom for a carbon atom next to a vacancy in the diamond lattice produces a fortuitous combination of magnetic and optical properties. In particular, individual NV centres can be identified by focusing green laser light onto a diamond crystal and observing a bright, localized red fluorescence.
Crucially for nanoMRI, the brightness of the NV fluorescence depends on its magnetic spin state, and the precession frequency of the NV spin state can also be measured with great precision. As a result, the NV centre essentially acts as an atomic-size magnetometer with nanotesla sensitivity. The study of individual NV centres was pioneered by Jörg Wrachtrup’s group at the University of Stuttgart in the mid-1990s, and the use of NV centres for nanoMRI detection was proposed in 2008 by Christian Degen, who is now at ETH Zurich.
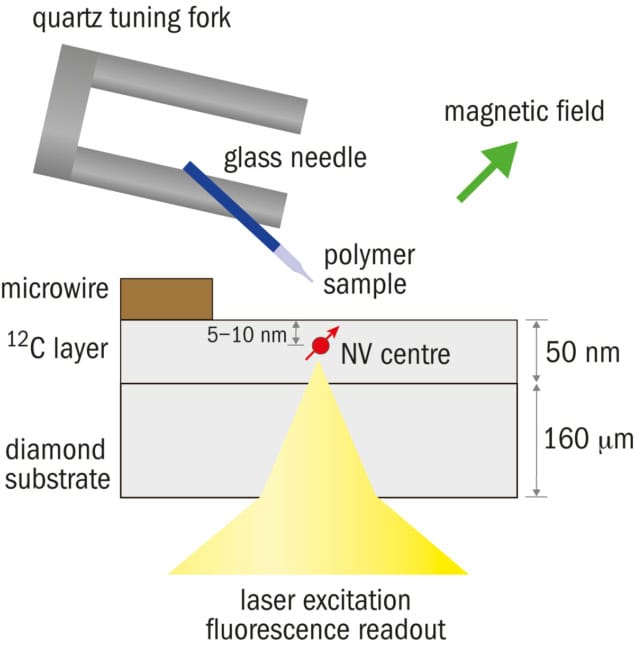
For nanoMRI applications, where the goal is to detect NMR signals from samples external to the diamond, the NV centre must be located as close to the diamond surface as possible. Near-surface NVs can be formed by ion implantation of nitrogen or by a “delta doping” process during chemical vapour deposition growth of the diamond layer.
One challenge in using near-surface NV centres is the reduction of the spin coherence – the regularity of the NV electron spin precession – because of poorly understood noise sources on the diamond surface. Recent advances in diamond surface preparation now allow NV centres with reasonably good characteristics – namely long coherence times and adequate photostability – to be formed just a few nanometres below the diamond surface.
The first demonstrations of NV-detected NMR from an external sample were achieved in 2012 by the Stuttgart group and, independently, by my group at IBM working in collaboration with David Awschalom, then at the University of California, Santa Barbara. In the Stuttgart work, a sequence of microwave pulses applied to the NV centre enabled the measurement of the oscillating magnetic field that naturally emanates from precessing hydrogen nuclei in an organic sample that was applied to the diamond surface.
In contrast, the IBM approach used a more active manipulation technique whereby the sample’s nuclear spins were flipped using radio-frequency magnetic fields. In both cases, the effect on the NV spin precession is detected via changes in the optical fluorescence of the NV centre. Although no imaging was involved in these initial demonstrations, it was clear from model calculations that the detected signals indeed originated from the hydrogen nuclei within a nanoscale sample volume.
Triple success
Recently these two groups and a third team at Harvard University independently succeeded in extending NV-based NMR detection into the realm of nanoMRI (Nature Nanotechnology 10 110, 125 and 129). Our group at IBM used a mechanical scanning approach to obtain a 2D hydrogen image of a polymer test sample that was scanned past a single NV centre, demonstrating a resolution of the order of 12 nm. The Stuttgart team used a similar approach whereby a patterned fluorocarbon sample was scanned over the NV centre and signals from both hydrogen and flourine-19 nuclei were detected. The third paper, from Ron Walsworth and colleagues at Harvard, used a dense layer of NV centres implanted just below the surface of a diamond substrate and employed a CCD camera to take a wide-field microscope image of NV fluorescence, achieving submicron MRI resolution without the need for mechanical scanning.
These initial demonstrations of NV-detected MRI are just a starting point, and we can expect great improvements in capability as researchers continue to improve the performance of NV-centre signal detection. The signal-to-noise ratio is currently limited by inefficiencies in the detection of the NV fluorescence, for example, but this could be overcome by incorporating recent innovations in diamond optical-waveguide techniques.
Another innovative idea, recently demonstrated by Mikhail Lukin’s group at Harvard, is to use “reporter” electron spins embedded in the sample molecule itself to act as an intermediary between the nuclear spins in the sample and the NV centre. By adding large field gradients to the NV detection system, we should be able to extend the technique to full 3D imaging, although ultimately it might require cryogenic temperatures to achieve the necessary scanning stability and NV coherence time required for a true molecular-structure microscope.
Both MRFM and NV-centre techniques have taken the resolution of MRI well beyond what anyone could have expected just a few years ago. It is clear that nanoMRI is no longer just a dream of a few far-out thinkers. Hopefully, the recent demonstrations will spur even more innovations to push the field forward.



