Important insights into how human bones grow and crystallize on an atomic scale have been made by Martin Andersson and colleagues at Sweden’s Chalmers University of Technology. Inspired by their use of 3D printing to try to mimic bone structures, the team determined that bone development is a thermodynamic process, independent of other biological systems within the body. Their discovery could soon be used to develop treatments for bone-related diseases, and could also aid developments of more advanced prosthetic implants.
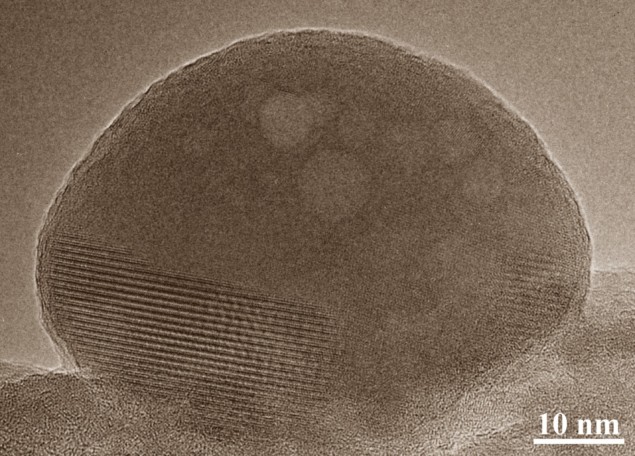
In our bones, new growth is initiated when spherical, disordered blobs of calcium phosphate are sent out from specialized cells to occupy the spaces in between strings of newly-formed collagen. When in place, these spheres transform from amorphous blobs into an orderly, crystalline substance known as apatite (see figure), which gives bone its unique mechanical properties. Exactly how this process plays out on a molecular scale had not been well understood.
The original goal of Andersson’s team was to create a 3D-printed material that accurately mimicked the mechanical properties of bone. Their goal was a material that would replace metal, plastic and other materials currently used in prosthetic implants, potentially improving patients’ mobility. Yet as the researchers optimized their material to imitate bone more precisely, they noticed their methods could also be used to explore the processes underlying bone development at unprecedented levels of detail.
Disorderly spheres
Using transmission electron microscopy to analyse their 3D printing process on an atomic scale, Andersson and colleagues observed for the first time how apatite is formed from disordered blobs of calcium phosphate. They found that when blobs reach the spaces in between collagen, nanometre-sized clusters of molecules migrate away from the blobs to occupy the lowest-energy locations on the bone’s growth front; eventually forming the solid, orderly structure of apatite.

Smart self-setting material improves bone implants
From their results, Andersson’s team showed that this process is driven by the presence of humidity in bone-forming regions. Intriguingly, this would suggest that bone formation is not a biological process; rather, it represents the thermodynamic response of calcium phosphate to its surroundings, independent of other systems within the body.
The team believes the insight could lead to more effective treatments for diseases including osteoporosis, where patients’ bones break down at a faster rate than new apatite can crystallize. The researchers hope their discoveries will allow the advantages of current and newly-proposed osteoporosis treatments to be evaluated more accurately. In addition, they will continue to assess the effectiveness of different substances for stimulating new bone growth, and for improving prosthetic implants.
The research is described in Nature Communications.



