As obesity rates continue to rise in many parts of the world, Cait MacPhee explains how soft-matter physicists could help reverse the trend by crafting “functional” foods that promote feelings of fullness and satisfaction
The developed world is getting fatter. In the UK, the incidence of obesity has almost quadrupled in the last 25 years; within the (mostly wealthy) countries that make up the Organisation for Economic Co-operation and Development (OECD), the majority of the population is now either overweight or obese. The reasons for this collective weight gain are manifold, and although sedentary lifestyles and the ready availability of calorie-dense foodstuffs are obvious contributors, they don’t appear to be the whole story. When we eat, our bodies respond with an incredibly complex hormonal process, one that takes into account not just what’s on our plates that day, but also what we have eaten in the past, and how much of it. Unfortunately, the outcome of this response is that, essentially, we train our bodies to get fat – and it doesn’t seem to be easy to train them to become thin again.
As an example, consider the process we use to recognize when we have eaten enough. The sensations of satiety (recognizing that we are full) and satiation (recognizing that we don’t want to eat again yet) stem from external social cues (such as plate size and portion size) and mechanical cues (a full stomach), but also from the response of our metabolism. When our gut detects the presence of fatty acids, sugars, amino acids or the breakdown products of proteins, it releases several known “satiety” hormones. These hormones help make us feel “full”, but the way our body releases them and how our brain reacts to them is complex and not yet fully understood. Our gastrointestinal tract is a complex organ: for example, the taste receptors in our mouths – the ones that enable us to tell whether foods are sweet, salty, sour, bitter and savoury (umami) – are also present in our stomach, small intestine and colon. It is not just our mouths that “taste” our food.
Injecting any of the satiety hormones temporarily decreases calorie intake in both lean and overweight humans, but unfortunately, these hormones are rapidly turned over in the body and the effect does not last to a second meal. Moreover, repeated doses do not lead to weight loss because our complex interconnected hormonal response adjusts to the presence of additional hormones. Thus the satiety hormones themselves probably do not represent good candidates for therapeutics. So what are the alternative prospects for intervention?
One option is to use social cues to promote satiety by, for example, decreasing the portion sizes of packaged foods: unsurprisingly, we eat less when less is available. We could also make foodstuffs denser and chewier, since we eat less when we eat slowly and chew more. However, given our fast-paced lifestyle and the prevalence of food-on-the-go, this may not be a realistic choice. And while we’re being pragmatic, we should also recognize that processed foods aren’t going to disappear overnight.
An alternative option is to re-engineer our food. That may sound extreme, but in some sense, it has already been done: many modern processed foods have been developed in response to consumer desires for creamier, richer, tastier foods. The challenge this time is to create tasty foods that can also deliver triggers for the release of satiety hormones directly to those tissues where they will do most good. This is where soft-matter physics enters, with the development of so-called “functional” foods.
Beyond mere nutrition
Functional foods are foods that offer added physiological effects beyond “normal” nutritional value and taste. Examples include bread, milk and orange juice enriched with vitamins; yoghurt with added “probiotic” bacteria; eggs with increased omega-3 fatty acids; and meat products with added fibrous material to decrease fat content. Functional foods can also make life easier for people with allergies and other health conditions: lactose-free and gluten-free foods are increasingly seen on supermarket shelves. But replacing ingredients in foods with healthier alternatives – or, in the case of satiety-promoting ingredients, adding new ingredients to established recipes – is not always easy. Food formulations are complicated and have often been developed empirically over time, so that removing an ingredient or adding a new one can have unexpected outcomes.
The task of understanding the structures of foodstuffs and food formulations falls squarely within the realm of soft-matter physics: the study of complex fluids containing dispersed structures such as bubbles, colloids, emulsions and/or polymers. The size of these structures ranges from nanometres to microns, similar to the length scale that you can detect on your tongue or in your mouth as you chew. Ice cream, for example, contains air bubbles, emulsions, ice crystals as colloidal particles, and proteins as both polymer and amphiphile (a molecule that has both water-soluble and water-insoluble parts). Chocolate consists of cocoa particles, sugar crystals and protein polymers in a continuous phase of cocoa butter. Beer foam is stabilized by polymeric biomolecular degradation products. And to a soft-matter physicist, pasta is basically just an amorphous carbohydrate in a glassy phase. All of these food formulations are beleaguered with biological complexity, but they are tractable and offer many approaches for delivering new, functional ingredients to the correct part of the body.
One possible approach for delivering satiety-promoting hormones to the gut would be to use emulsions – droplets of one liquid (the “dispersed phase”) suspended in another (the “continuous phase”). Many different processed foods contain emulsions: salad dressings, for example, are oil droplets suspended in a water-rich phase, while butter and margarine are water droplets suspended in an oil-rich phase. The emulsion droplets can be stabilized by amphiphiles (emulsifiers) to prevent coalescence, or they can be suspended in “texture modifiers” that thicken or gel the continuous phase, preventing droplets colliding. As these systems consist of aqueous, oily and amphiphilic phases, they can also accommodate many different functional ingredients to promote satiety, potentially simultaneously. By combining and processing emulsions appropriately it is possible to create a range of textures, from pastes and gels to freely flowing liquids. Some can even be dried and added as a powdered ingredient. Importantly, emulsions can be created from a range of food-grade ingredients using relatively simple and energy-efficient processing methods. Emulsions do have some limitations, however, many of which are encountered during food preparation. High and low temperatures (such as those encountered when cooking, chilling or freezing food), vigorous mixing, and changes in pH can all cause the droplets in emulsions to become unstable. This creates problems for would-be developers of functional foods. If oil-in-water emulsions are destabilized, for example, an oil-rich phase can be formed in or on the foodstuff – an undesirable product behaviour known as “oiling out”. The stability of the emulsion droplets can be improved by coating them with a thin solid surface layer built up from charged polyelectrolytes such as carbohydrate polymers or proteins. This layer can be chosen so as to dissolve or become porous only under certain conditions of pH and/or salt, for targeted release of satiety factors at certain places in the gut.
The surface layer does not have to be thin and does not have to be formed from polymer layers: cellulose particles or even smaller emulsion droplets that are themselves stabilized with a surface layer of protein can pack around an emulsion droplet, forming what is known as a Pickering emulsion (figure 1). The use of such emulsions, however, depends on the food: emulsions of this size can be detected in the mouth, and in semi-liquid preparations such as yoghurt, they give an unpleasant gritty consistency.
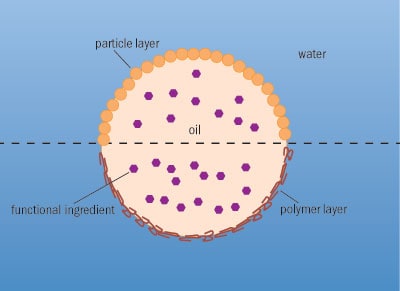
A further example of emulsion technology is an emulsion droplet captured within an emulsion – a hierarchical structure known as a “multiple emulsion”. Examples might be oil droplets within a water droplet suspended in an oil continuous phase, or water droplets inside an oil droplet in a primarily aqueous formulation. Multiple emulsions are useful for “trapping” volatile ingredients, preventing them from diffusing out of the food by surrounding them with a medium through which they cannot pass. They are also useful for trapping ingredients that might otherwise taste bitter, by preventing their release in the mouth. Finally, multiple emulsions can protect fragile ingredients from the surrounding environment, preventing unwanted chemical reactions that may lead to food spoilage.
Although emulsions offer many opportunities for encapsulating and delivering functional ingredients, unfortunately the development of functional foods is a little more complicated than simply picking the right emulsion off the shelf. Our body responds differently to emulsion particles of different sizes and compositions. Some studies have shown that smaller emulsion droplets deliver both calories and satiety, while larger ones deliver the calories but with a muted satiating effect. The location where the emulsion is processed within the body also matters: if oily emulsions are unstable in the acidic environment of the stomach, the stomach becomes lined with fat, which seems to decrease satiety. But the breakdown products of fats are satiety-inducing factors themselves. Until we understand this complex interplay between the processing conditions that make emulsions larger or smaller, our enjoyment of the resulting foods in terms of taste and texture, their digestion in our gut, and the subsequent release of satiety hormones, we are trying to hit a moving target.
Protein power
Proteins are also attractive ingredients for food structuring. Compared to fats, they have two important advantages: a lower calorie density and an increased intrinsic satiety. Simple changes in temperature or pH can cause proteins to form filamentous (transparent) or particulate (opaque) gels that can be used to give foods texture. As mentioned above, these texture-modifying gels can be used to prevent or slow the emulsion droplets coalescing, by thickening the surrounding medium. Alternatively, by controlling how proteins stick together through simple changes in temperature and/or pH it is possible to form protein particles that act similarly to emulsions. Like emulsions, these can be used to encapsulate and promote the slow release of satiety-promoting ingredients, while also giving foods a “creamy” texture without the addition of fats or oils.
Similar effects can be achieved with carbohydrate polymers such as starch, cellulose, chitosan, alginate and gums including guar gum and xanthan. Many of these are used to encapsulate bacteria as probiotics; however, careful processing is required to make these capsules resistant to the acid environment of the stomach, which could otherwise destroy the bacteria before they reach the intestine. Many of these polysaccharides are also used as thickening agents, and a number of them cannot be digested, so do not directly contribute to calorie content. Some of these “dietary fibres” are also intrinsically satiety-inducing, perhaps by adding bulk or increasing water content to foods and giving rise to a mechanical feeling of fullness, but possibly by triggering the release of satiety hormones following fermentation in the intestine.
Foods for the future
Re-engineering processed foods to have a lower energy density is, in theory, a relatively straightforward matter: all you need to do is replace high-calorie ingredients (such as fats) with low-energy-density alternatives (such as dietary fibre). However, doing so typically has an impact on consumer satisfaction, as both the taste and texture of the food are affected. Food physics offers the opportunity to get around this dilemma by creating new products that mimic the texture and taste of unhealthy but well-loved foods, with added components that, when released in a controlled fashion at specific sites within the body, improve our feeling of satisfaction. Clever food processing also offers the potential for decreasing the amount of salt and sugar in processed foods, at least where they are added for reasons other than taste or nutrition.
In an ideal world in which processed foods were eaten rarely or not at all, such solutions would not be necessary. However, in a world where 90% of Americans purchase convenience foods and more than 50% of the calorie intake in the UK comes from ultra-processed and energy-dense foods, such advances are clearly desirable. Perhaps clever physics may yet help us stem the developed world’s obesity crisis.



