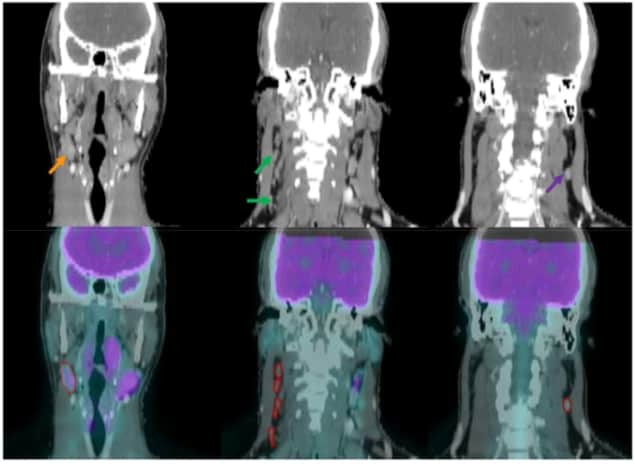
Researchers have developed a hybrid predictive model to improve the identification of metastatic lymph nodes in individuals with head-and-neck cancer. Classifying nodes in PET/CT images, the model combines radiomics and deep learning and outperformed two existing techniques. By tailoring treatments more accurately to the individual, it has the potential to improve disease control and prevent over-treatment (Phys. Med. Biol. 10.1088/1361-6560/ab083a).
“Critical decisions often hinge on whether a patient has involved lymphadenopathy, such as treating with primary surgery or radiation, and whether to add chemotherapy to radiation treatment,” says David Sher, study co-author and radiation oncologist at University of Texas Southwestern Medical Center. “Radiation planning requires accurate diagnostics, since the optimum radiation dose to the neck is intimately related to the presence of lymph node metastases.”
Currently, clinicians typically assess images of lymph nodes by eye. Malignant nodes that are large and exhibit high PET tracer uptake are easy to spot, but smaller, less reactive ones are harder to pick out. “The delineation of questionable or suspicious lymph nodes strongly depends on the physicians’ clinical experience,” says first author, Liyuan Chen, also based at the University of Texas. “Machine-learning algorithms can potentially make clinical practice in malignant lymph node identification more accurate and consistent across physicians and facilities.”
The hybrid model classifies lymph nodes as normal, suspicious or involved. It is the first of its kind to be applied to the problem. In the current study, the group trained and tested the model using PET/CT images from 59 patients, participants in the separate INFIELD radiotherapy clinical trial at the Dallas centre. The images contained 236 lymph nodes (22 involved, 27 suspicious and 17 normal); 170 were used for training and 66 were used to test each of the two model components.
The researchers’ many-objective radiomics model extracts 257 handcrafted features — pre-defined metrics characterizing aspects including geometry, intensity and texture — from every image. It comprises a support vector machine to generate a predictive model, coupled with an overarching optimization algorithm. The algorithm uses six objective functions, two per type of lymph node, to test model parameters and subsets of features, identifying the combination of the two that classifies the nodes most accurately.
The deep-learning component is a 3D convolutional neural network (CNN). Here, the most optimal, abstract features for node classification are identified by the network automatically. Outputs from the CNN and radiomics models are then fused using evidential reasoning.
By fusing information from different imaging modalities and classifiers, the approach is, in principle, more stable than techniques using a single modality or classifier. “We expect that the hybrid model can perform more consistently when applied to different imaging data sets,” says senior author Jing Wang.
The hybrid model’s performance was compared against XmasNet, a recently developed CNN, and a conventional radiomics model using only one objective function. It was more accurate when analysing both PET and CT data (0.88 versus 0.81 for XmasNet and 0.75 for conventional radiomics). This was matched, however, by the performance of the CNN component alone. Slightly higher accuracy values were obtained with the hybrid approach when CT or PET images were analysed separately. The authors anticipate future studies will demonstrate the technique’s stability over non-hybrid approaches.

The group is currently working on further validations, as well as investigating more advanced neural network architectures for the model. These include an analysis of head-and-neck patients imaged prior to surgery, whose preliminary results are “very promising,” according to Wang. A clinical trial is also set to start this year.
The researchers envisage their technique could be integrated into the treatment planning process in radiotherapy clinics, with the clinician-contoured lymph nodes providing the input for the model. “Our model will generate malignancy probability for each lymph node, which will aid in physician’s decision whether to include the lymph node as part of clinical target volume,” Wang tells Physics World.



