Mammography can reduce breast cancer deaths by detecting tumours at their earliest, most treatable stages. However, up to 60% of women screened with mammography over 10 years have at least one false-positive result, necessitating additional diagnostic imaging and, in many cases, biopsies.
To reduce the number of unnecessary breast biopsies, a US research team has devised a novel image analysis technique that uses mammography to determine the biological tissue composition of a suspicious breast mass (Radiology 10.1148/radiol.2018180608).
“The call-back rate with mammography is much higher than ideal,” explains first author Karen Drukker from the University of Chicago. “There are costs and anxiety associated with recalls, and our goal is to reduce these costs but not miss anything that should be biopsied.”
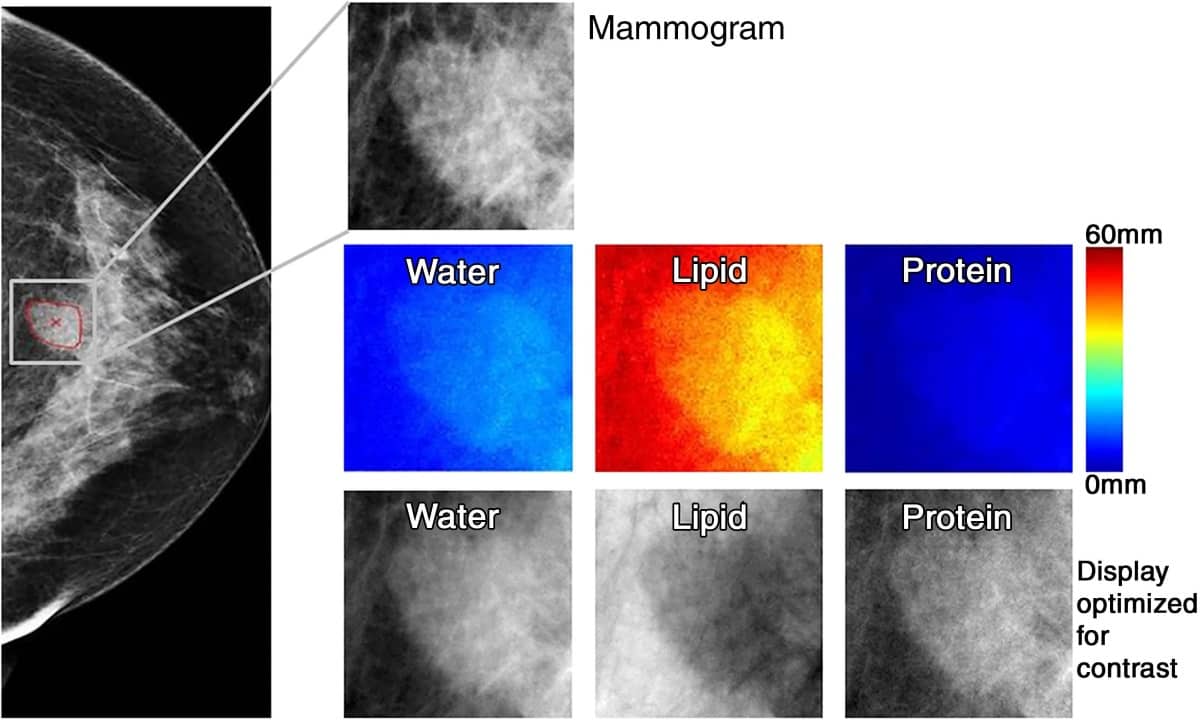
Drukker and colleagues employed three-compartment breast (3CB) imaging, a dual-energy mammography technique that does not require a contrast agent and delivers only 10% higher dose than standard mammograms. By measuring the water, lipid and protein tissue composition throughout the breast, 3CB might provide a biological signature for a tumour. For instance, more water in the tumour tissue might indicate angiogenesis, an early sign of cancer development.
For this prospective study, the team acquired dual-energy mammograms from 109 women with suspicious breast masses that typically would require further investigation, immediately prior to biopsy. The ensuing biopsies showed that 35 masses were invasive cancers, while the other 74 were benign.
The researchers derived 3CB images from these dual-energy mammograms and calculated the water, lipid and protein thickness at each pixel. They then combined this analysis with mammography radiomics, which uses artificial intelligence algorithms to analyse features and patterns in images.
The combination of 3CB image analysis and radiomics improved the positive predictive value in suspicious breast masses from 32% for conventional diagnostic digital mammography to 49%, with a sensitivity of 97%. This corresponds to a reduction in biopsies of almost 36%.
Drukker says that this combined 3CB–radiomics approach has the potential to play an increasingly prominent role in breast cancer diagnosis and perhaps also in screening. She notes that 3CB can easily be added to mammography without requiring extensive modifications of existing equipment. “The patient is already getting the mammography, plus we get all this extra information with only a 10% additional dose of radiation,” she points out.
This approach is still experimental and further work is needed to make it available to patients. The researchers plan to study how the combined 3CB–radiomics approach will help radiologists make their final determinations. They also want to study its use with digital breast tomosynthesis, which reduces the problem of overlapping breast tissue inherent to regular mammography. A tumour’s unique water-lipid-protein signature might be even clearer with tomosynthesis, says Drukker.



