A new atomic force microscopy (AFM) technique, which would allow users to precisely detect and map the charge distribution within molecules, has been developed by researchers in Europe. The team has used its method to demonstrate the difference in bond polarity between two structurally identical but chemically distinct molecules. It hopes the technique could someday be used to map charge movement within photovoltaic materials – something that could potentially help to improve solar cells.
In 1991 a variation of AFM – dubbed Kelvin probe force spectroscopy (KPFS) – was invented, and is used to measure the charge distribution on a surface. As a nanoscale tip oscillates at a tiny distance from the surface, a bias voltage is applied between the two. The electrostatic interaction with the surface pushes and pulls on the tip, changing its oscillation frequency. By measuring the frequency at various points, one can calculate the potential difference between tip and surface, and thereby infer the surface distribution of electronic charge.
Chemically attached
In 2009 Leo Gross and colleagues at IBM Research Zurich in Switzerland showed that, by “functionalizing” the tip – making it chemically reactive by attaching a certain molecule to it – they could use AFM to obtain images of individual atoms within molecules. However, to measure the distribution of charge within individual chemical bonds, one needs to perform KPFS with the tip at sub-nanometre distances from the surface. Here, previously unexplained artefacts appear in the images, making KPFS unreliable.
In the new research, physicist Jascha Repp of the University of Regensberg in Germany and colleagues analysed the forces acting on the tip, to better understand the artefacts that appear in the images. The interactions between tip and surface have multiple origins – apart from the electrostatic force at play, there is also repulsion that arises due to Pauli’s exclusion principle, which dictates that two identical fermions cannot simultaneously occupy the same quantum state. The closer the tip gets, the more significant is the Pauli repulsion. The catch is that, as the potential difference is raised and lowered, the molecules on the surface – and more significantly, on the tip – are distorted, changing the tip-to-surface distance, as well as the consequent significance of the Pauli repulsion, leading to artefacts in KPFS images.
Tip-top solution
Repp’s team has now devised a solution, however. The researchers measured the rate of change of the tip’s oscillation frequency with height, as they brought it closer to the surface. This frequency shift was due to electrostatic interaction, Pauli repulsion and other forces such as van der Waals attraction. They then altered the potential difference between tip and surface, and repeated the process. The electrostatic interaction has a different mathematical dependence on distance, from that of the other forces. Therefore, by comparing how the total force varied with distance at both voltages, the researchers were able “to better disentangle all these different contributions and…extract better only the electrostatic contribution”, explains Repp.
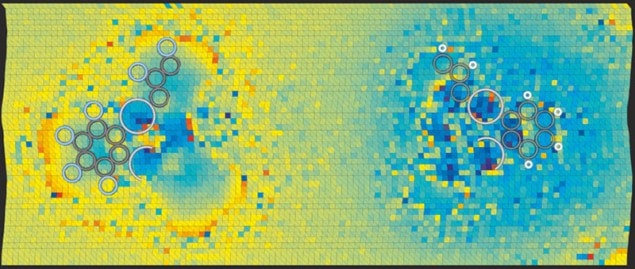
To demonstrate their technique, the researchers examined several simple molecules. In all cases, the results were closer than traditional KPFS to theoretical predictions. For example, they looked at the structurally identical molecules F12C18Hg3 and H12C18Hg3, showing for the first time that the C–H bonds in the first compound were polarized with the negative charge on the carbon atom, whereas the C–F bonds in the second were polarized the opposite way, as predicted. The team hopes that its method could someday be used to study changes in charge distribution as molecules become excited, thereby helping to optimize organic photovoltaic compounds.
“It’s an important piece of work and it’s given us key insights into electrostatic forces,” says physicist Philip Moriarty at the University of Nottingham. “The only reservation I have – and the authors themselves state this reservation – is that it involves quite a number of approximations. Nonetheless, the comparison with traditional KPFS data is pretty compelling.” He notes that another recently unveiled technique for extracting the same information by functionalizing the tip with a quantum dot, achieves “comparable” resolution. Moriarty and Gross both believe the two techniques are complementary. “The other technique is very sensitive at longer distances where the lower-order electrostatic multipoles become more dominant,” Gross says. “When you do scanning probe microscopy, you often want high lateral resolution, and therefore it’s important to be able to interpret results that you get at close tip-sample distances. That’s the region which the current paper addresses.”
The research is published in Physical Review Letters.